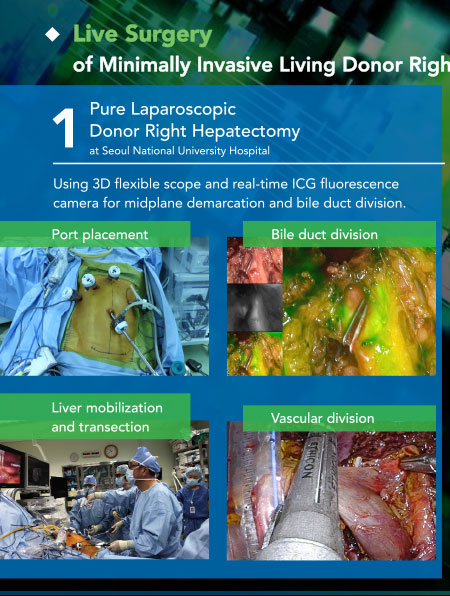
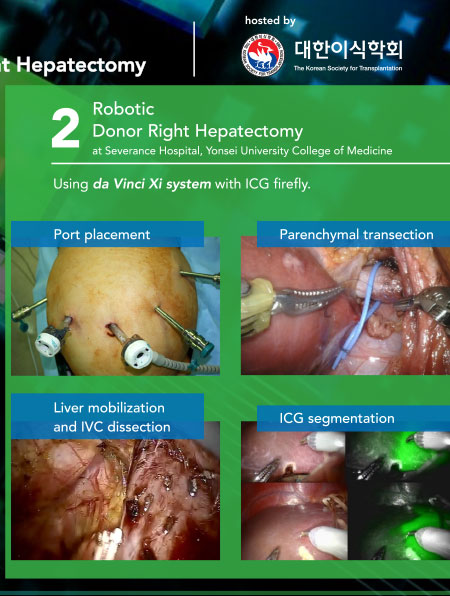
TTS 2020 Virtual Congress - Live Surgery
TTS 2020 VIRTUAL CONGRESS
Live Surgery

TRANSPLANTATION - HIGHLIGHTED ARTICLE
Dr. Jeremy R. Chapman, Editor-in-Chief, Transplantation
eHealth Interventions for Solid Organ Transplant Recipients: A Systematic Review and Meta-analysis of Randomized Controlled Trials
Tang J, James L, Howell M, et al.
Transplantation: August 2020 - Volume 104 - Issue 8 - p e224-e235
Every hospital affected by the current pandemic has moved to ehealth solutions for care to keep patients out of the hospital – not only to stop them being exposed to the virus needlessly but also to free up capacity in the hospitals. But do we know what impact that has – is it bad for patient care? Does it reduce non-adherence and improve compliance with care? In this timely systematic review we can see the data available today and plan what we should be trying to discover in this time of necessity. The answer? There have been 21 trials from 6 countries involving 2114 participants and compared with standard care, eHealth interventions improved medication adherence and self-monitoring behaviour up to 12 months post transplant. Nine trials reported harms but sadly the quality of evidence was generally low to very low. An important read for all who think that this headlong rush to eHealth is efficient and good.
TRANSPLANTATION - WEEK'S MOST DOWNLOADED PAPER
«HOT OFF THE PRESS»
RECENT PUBLICATIONS IDENTIFIED BY TTS EDUCATION COMMITTEE ON COVID-19
A dynamic COVID-19 immune signature includes associations with poor prognosis
Adam G. Laing et al.
Nature Medicine, August 17, 2020; https://doi.org/10.1038/S41591-020-1038-6
This article investigated peripheral blood immune signature across 63 hospital-treated patients with COVID-19. The signature includes discrete changes in B and myelomonocytic cell composition, profoundly altered T cell phenotypes, selective cytokine/chemokine upregulation and SARS-CoV-2-specific antibodies. Some signature traits identify links with other settings of immunoprotection and immunopathology; others, including basophil and plasmacytoid dendritic cell depletion, correlate strongly with disease severity; while a third set of traits, including a triad of IP-10, interleukin-10 and interleukin-6, anticipate subsequent clinical progression.
Artificial intelligence–enabled rapid diagnosis of patients with COVID-19
Xueyan Mei et al.
Nature Medicine, 26, pages1224–1228(2020)
This article used used artificial intelligence (AI) algorithms to integrate chest CT findings with clinical symptoms, exposure history and laboratory testing to rapidly diagnose patients who are positive for COVID-19. Among a total of 905 patients tested by real-time RT–PCR assay and next-generation sequencing RT–PCR, 419 (46.3%) tested positive for SARS-CoV-2. In a test set of 279 patients, the AI system achieved an area under the curve of 0.92 and had equal sensitivity as compared to a senior thoracic radiologist. The AI system also improved the detection of patients who were positive for COVID-19 via RT–PCR who presented with normal CT scans, correctly identifying 17 of 25 (68%) patients, whereas radiologists classified all of these patients as COVID-19 negative. When CT scans and associated clinical history are available, the proposed AI system can help to rapidly diagnose COVID-19 patients.
In-depth virological assessment of kidney transplant recipients with COVID-19
Benotmane et al.
AJT 2020, doi:10.1111/AJT.16251
This article examined hospitalized kidney transplant recipients with non-severe (n = 21) and severe (n =19) COVID-19. SARS-CoV-2 nasopharyngeal and plasma viral load and serological response were evaluated based on outcomes and disease severity. Ten recipients (25%) displayed persistent viral shedding 30 days after symptom onset. The SARS-CoV-2 viral load of the upper respiratory tract was not associated with severe COVID-19, whereas the plasma viral load was associated with COVID-19 severity (p=0.010) and mortality (p=0.010). All patients harbored antibodies the second week after symptom onset that persisted for two months.
Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19
Ling Li et al.
JAMA. 2020;324(5):460-470. doi:10.1001/jama.2020.10044
This is an open-label, multicenter, randomized clinical trial performed in 7 medical centers in Wuhan, China, from February 14, 2020, to April 1, 2020,to evaluate the efficacy and adverse effects of convalescent plasma therapy for patients with COVID-19. Of 103 patients who were randomized (median age, 70 years; 60 [58.3%] male), 101(98.1%) completed the trial. Clinical improvement occurred within 28 days in 51.9% (27/52) of the convalescent plasma group vs 43.1%(22/51) in the control group (difference, 8.8% [95%CI,−10.4%to 28.0%]; hazard ratio [HR], 1.40 [95%CI, 0.79-2.49]; P = .26). There was no significant difference in 28-day mortality (15.7% vs 24.0%; OR, 0.59 [95%CI, 0.22-1.59]; P = .30) or time from randomization to discharge(51.0% vs 36.0% discharged by day 28; HR, 1.61 [95%CI, 0.88-2.95]; P = .12). Convalescent plasma treatment was associated with a negative conversion rate of viral PCR at 72 hours in 87.2% of the convalescent plasma group vs 37.5% of the control group (OR, 11.39 [95%CI,3.91-33.18]; P < .001).
Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience
Michael J. Joyner et al. for the US EAP COVID-19 Plasma Consortium
medRxiv preprint doi: https://doi.org/10.1101/2020.08.12.20169359.
This study investigated efficacy of COVID-19 convalescent plasma. The 35,322 transfused patients included. There were high proportion of critically-ill patients, with 52.3% in the intensive care unit (ICU) and 27.5% receiving mechanical ventilation at the time of plasma transfusion. The seven-day mortality rate was 8.7% [95% CI 8.3%-9.2%] in patients transfused within 3 days of COVID-19 diagnosis but 11.9% [11.4%-12.2%] in patients transfused 4 or more days after diagnosis (p<0.001). Similar findings were observed in 30-day mortality (21.6% vs. 26.7%, p<0.0001). The pooled relative risk of mortality among patients transfused with high antibody level plasma units was 0.65 [0.47-0.92] for 7 days and 0.77 [0.63-0.94] for 30 days compared to low antibody level plasma units.
Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes. Interim Analysis of 2 Randomized Clinical Trials
Xia et al.
JAMA. Published online August 13, 2020. doi:10.1001/jama.2020.15543
This was an interim analysis of 2 randomized placebo-controlled trials. In 96 healthy adults in a phase 1 trial of patients randomized to aluminum hydroxide (alum) only and low, medium, and high vaccine doses on days 0, 28, and 56, 7-day adverse reactions occurred in 12.5%, 20.8%, 16.7%, and 25.0%, respectively; geometric mean titers of neutralizing antibodies at day 14 after the third injection were 316, 206 and 297 in the low-, medium-, and high-dose groups, respectively. In 224 healthy adults randomized to the medium dose, 7-day adverse reactions occurred in 6.0% and 14.3% of the participants who received injections on days 0 and 14 vs alum only, and 19.0% and 17.9% who received injections on days 0 and 21 vs alum only, respectively; geometric mean titers of neutralizing antibodies in the vaccine groups at day 14 after the second injection were 121 vs 247, respectively. In conclusion, this inactivated COVID-19 vaccine had a low rate of adverse reactions and demonstrated immunogenicity, but longer-term assessment of safety and efficacy will require phase 3 trials.
CORONAVIRUS (COVID-19) UPDATES
INVITATION TO PARTICIPATE

Dear Members,
Researchers at McGill University are currently conducting a study entitled LIST-COVID-19 to study the impact of the COVID-19 pandemic on transplantation activity, volumes and immunosuppression practices on a global scale. The study will also help better understand the current and anticipated risks to transplantation during the pandemic and inform clinical practice during the “ramp-up” phase. The Transplantation Society is supporting the project, as well as several National and International Societies.
They are looking for physicians who take care of patients with a solid organ transplant to take a 10-15 minute survey on behalf of their center and program. Please note this is not a patient registry and no specific patient-level data is being collected. Please be assured they will only perform a collective analysis of the survey questions and no personal information identifying the participants will be divulged. The survey responses will be confidential. If you are interested in participating, please contact them at the emails listed below.
Investigators: Shaifali Sandal, MD and Marcelo Cantarovich, MD
Contact: shaifali.sandal@mcgill.ca and marcelo.cantarovich@muhc.mcgill.ca
The Transplantation Society (TTS) and our journal Transplantation have developed online resources to keep you informed on the Coronavirus (COVID-19) outbreak.
- TTS Coronavirus (COVID-19) Dashboard
www.tts.org/covid-19 - Transplantation Global Transplantation COVID Report
www.tts.org/txjcovid19
We are also requesting contributions and news from the transplant community to be sent to covid-19@tts.org for inclusion on our resources page.
In this dashboard, you will find links to TTS and other global and regional resources, as well as interactive maps, publications and webinars. We encourage you to explore this dashboard and share with your colleagues.
Editors and contributors to Transplantation have shared their thoughts on how they are dealing with the current crisis. While we understand that the information of today may be quite different tomorrow in this fast-moving pandemic, this report will open our forum of an international exchange on COVID for the transplant community.
Website - www.tts.org/txjcovid19
Please send your own contributions and news to covid-19@tts.org for inclusion on our resources page.
IN THE NEWS
DISPARITIES IN ACCESS TO HCV POSITIVE-DONOR/HCV NEGATIVE-RECIPIENT TRANSPLANT
Aug. 11 - Following successful pilot trials of transplanting organs from hepatitis C virus (HCV) viremic donors into HCV-negative recipients, there has been an expansion of the practice. Direct-acting antiviral (DAA) therapies are costly, creating barriers to insurance coverage approvals, particularly in transplantation from HCV positive donors to HCV-negative recipients, due in part to off-label treatment of acute HCV following intentional HCV transmission.
DUAL SURGERY BOOSTS HEART TRANSPLANT CHANCES FOR PATIENTS WITH OBESITY
Aug. 12 - Obese patients with heart failure are more likely to meet eligibility criteria for a heart transplant if they first undergo bariatric surgery and receive a left ventricular assist device, according to a study conducted by Jefferson Health.
OUTCOMES AMONG RECIPIENTS OF OLDER HYPERTENSIVE LIVING DONOR KIDNEYS
Aug. 11 - There have been data reported supporting the long-term safety of nephrectomy in donors with hypertension. However, according to A. Sharfuddin and colleagues at Indiana University School of Medicine, Indianapolis, there are few long-term data on outcomes among recipients of kidneys from donors with hypertension.
DONATION AND TRANSPLANTATION ACTIVITY IN THE UK DURING THE COVID-19 LOCKDOWN
Aug. 15 - As of May 14, 2020, over 11 000 patients with COVID-19 in the UK were admitted to intensive care units (ICUs), with a median length of stay of 9 days. The COVID-19 pandemic had the immediate effect of severely reducing living and deceased organ donation and transplantation activity, as happened in other countries.
OSTEOCHONDRAL AUTOGRAFT TRANSPLANTATION MAY BE AN EFFECTIVE TREATMENT FOR TALAR LESIONS
Aug. 19 - Osteochondral autograft transplantation has shown positive clinical outcomes in the treatment of medium-sized osteochondral lesions of the talus, according to a study by researchers at Hospital for Special Surgery.
UPCOMING MEETINGS AND ANNOUNCEMENTS
TTS BUSINESS MEETING

In accordance with the TTS By-Laws, a Business Meeting will be held shortly after the close of the TTS 2020 Virtual Congress. The meeting will be help on Sunday, September 20, at 8 AM Montreal time and will last approximately one hour. The agenda will include an update on the TTS finances, the announcement of the TTS 2024 Congress location, a presentation for the TTS 2022 Congress in Buenos Aires, the announcement of the 2020-2022 Councilors and Officers and the official change of TTS Presidency. All members are invited to participate.
As this meeting will be virtually, we will send all members a link to join this meeting shortly before the meeting takes place.
Sincerely,
John Fung
Secretary, The Transplantation Society
18th Asian Pacific Congress of Nephrology (APCN)
SPLIT Virtual Annual Meeting
IPITA-IXA-CTRMS Joint Congress
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada