2017 - IPITA
This page contains exclusive content for the member of the following sections: TTS, IPITA. Log in to view.
Islet Isolation, Culture & Preconditioning 2
31.8 - Glucose-stimulated insulin secretion in human islets: static incubation versus dynamic perifusion techniques
Presenter: Francois, Pattou, Lille, France
Authors: Valéry Gmyr, Caroline Bonner, Ericka Moermann, Anais Codeville, Nathalie Delalleau, Julien Thévenet, Gianni Pasquetti, Sandrine Belaich, Isanga Aluka, Bruno Lukowiak
Glucose-stimulated insulin secretion in human islets: static incubation versus dynamic perifusion techniques
V. Gmyr1, C. Bonner1, E. Moermann1, A. Codeville1, N. Delalleau1, J. Thévenet1, G. Pasquetti1, S. Belaich1, I. Aluka1, B. Lukowiak1.
1Translational Research for Diabetes, INSERM UMR1190 - European Genomic Institute for Diabetes, University of Lille, Lille, France, ; 2Unit of Endocrinology and Metabolism, Faculty of Medicine, University of Louvain, Brussels, Belgium,
Aims of study: The success of islet transplantation is limited by islet isolation procedure, heterogeneity of the donors, the number of islets required for grafting and the physiological quality of human islet preparations. Adequate secretion of insulin in response to blood glucose variations is the ultimate, most sought-after property of transplanted islets. Choosing the optimal method for evaluating the functional quality of islet preparations in vitro is thus important. In this study we compared glucose-stimulated insulin secretion (GSIS) by human islet preparations during static incubations and dynamic perifusions.
Methods: Islets were isolated and purified from 16 human pancreata according to the automated method of Ricordi et al. For each preparation, the islet quality was evaluated by measuring apoptosis, ATP content and viability. GSIS during static incubations and dynamic perifusions was compared by calculating a stimulation index (SI): ratio of insulin responses to high versus low glucose.
Results: No correlations were found between GSIS measured during static islet incubations and islet apoptosis (r2=0.0003, P=0.947), viability (r2=0.163, P=0.121) or ATP content (r2=0.032, P=0.504). GSIS measured during perifusions was (weakly) negatively correlated with islet ATP content (r2= -0.379, P=0.011). The graph shows no correlation between GSIS measured by the two techniques (r2=0.0002, P=0.955). In perifused islets, GSIS consistently displayed a biphasic pattern and the SI was much greater (average 8.1, range 3.8-17.6) than in incubated islets (average 1.55, range 0.51 – 3.05) (P<0.0001). The large range of insulin responses in perifused islets (4.5-fold) illustrates the functional heterogeneity of human islet preparations. 16/16 preparations shown a SI >1 in perifusion whereas 12.5% of preparation had a SI ≤1 in static incubation.
Conclusion: A dynamic system of perifusion is thus much superior to a static incubation system to assess GSIS in human islets. Classic parameters of islet quality did not predict the amplitude of the insulin response. The paradoxical negative correlation with the islet ATP content might be due to the often neglected existence of stable pools of nucleotides in insulin granules.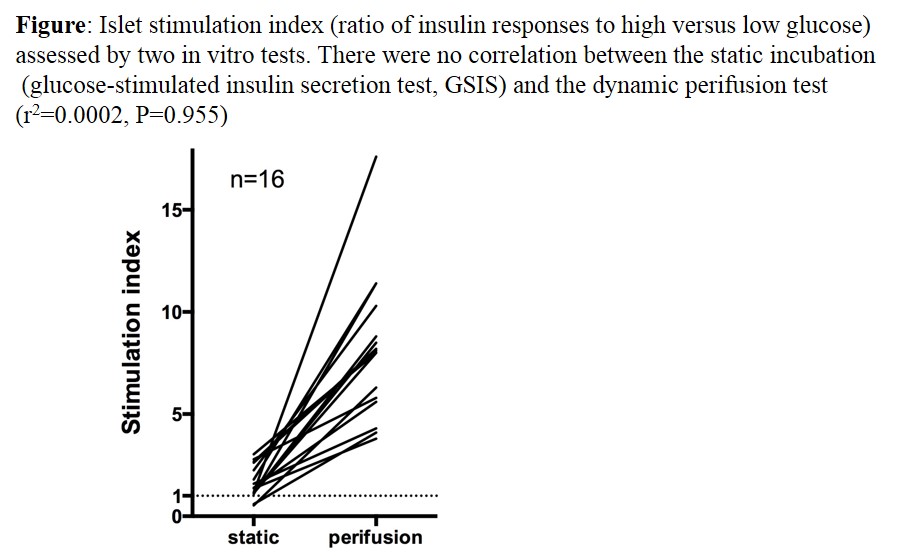
Important Disclaimer
By viewing the material on this site you understand and accept that:
- The opinions and statements expressed on this site reflect the views of the author or authors and do not necessarily reflect those of The Transplantation Society and/or its Sections.
- The hosting of material on The Transplantation Society site does not signify endorsement of this material by The Transplantation Society and/or its Sections.
- The material is solely for educational purposes for qualified health care professionals.
- The Transplantation Society and/or its Sections are not liable for any decision made or action taken based on the information contained in the material on this site.
- The information cannot be used as a substitute for professional care.
- The information does not represent a standard of care.
- No physician-patient relationship is being established.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
