Welcome to the
International Xenotransplantation Association
President's Letter - October 2025

Dear Friends,
I wanted to share my appreciation for the many people who made the 2025 Congress such a great success. First, the local organizing committee did a wonderful job updating all of us on exciting new science and clinical trials in xenotransplantation. The rooms were full right to the end. Thank you! As you may not know, “our staff” is really the staff of the Transplantation Society (TTS) who work night and day to make the meetings work – and provided their usual meticulous performance with unusual grace. We also thank our Past-President Muhammad Mohiuddin for his leadership and vision over the past two years.



Immediate Past-President's Message - IXA 2025
Dear Friends,
The congress of the International Xenotransplantation Association (IXA) ended on Friday in Geneva, Switzerland, and was a resounding success. As the President of the Association at that time, I was humbled by the extraordinary attendance from start to finish. This enthusiasm was achieved through the high quality of xenotransplantation work, even unpublished, that was openly shared by leaders in the field for the benefit of clinical translation of this life-saving option of xenotransplantation.
The participation of over 400 attendees from 21 countries in this meeting demonstrated renewed interest in xenotransplantation, fueled by the granting of permissions for clinical trials.
I am confident that the lessons learned from this meeting will be effectively applied to advance the field further. Thanks again to the membership for making it possible. As I welcome our new president, Jay Fishman, and the council, I hope IXA will continue to provide leadership in this field by developing guidance for investigators and for regulatory agencies worldwide.
As we drafted the slogan for this meeting, “We only speak Xeno,” IXA will continue to lead the xenotransplantation field in making it a viable and affordable option for overcoming the worldwide organ shortage.
Muhammad Mansoor Mohiuddin
Immediate Past-President, IXA
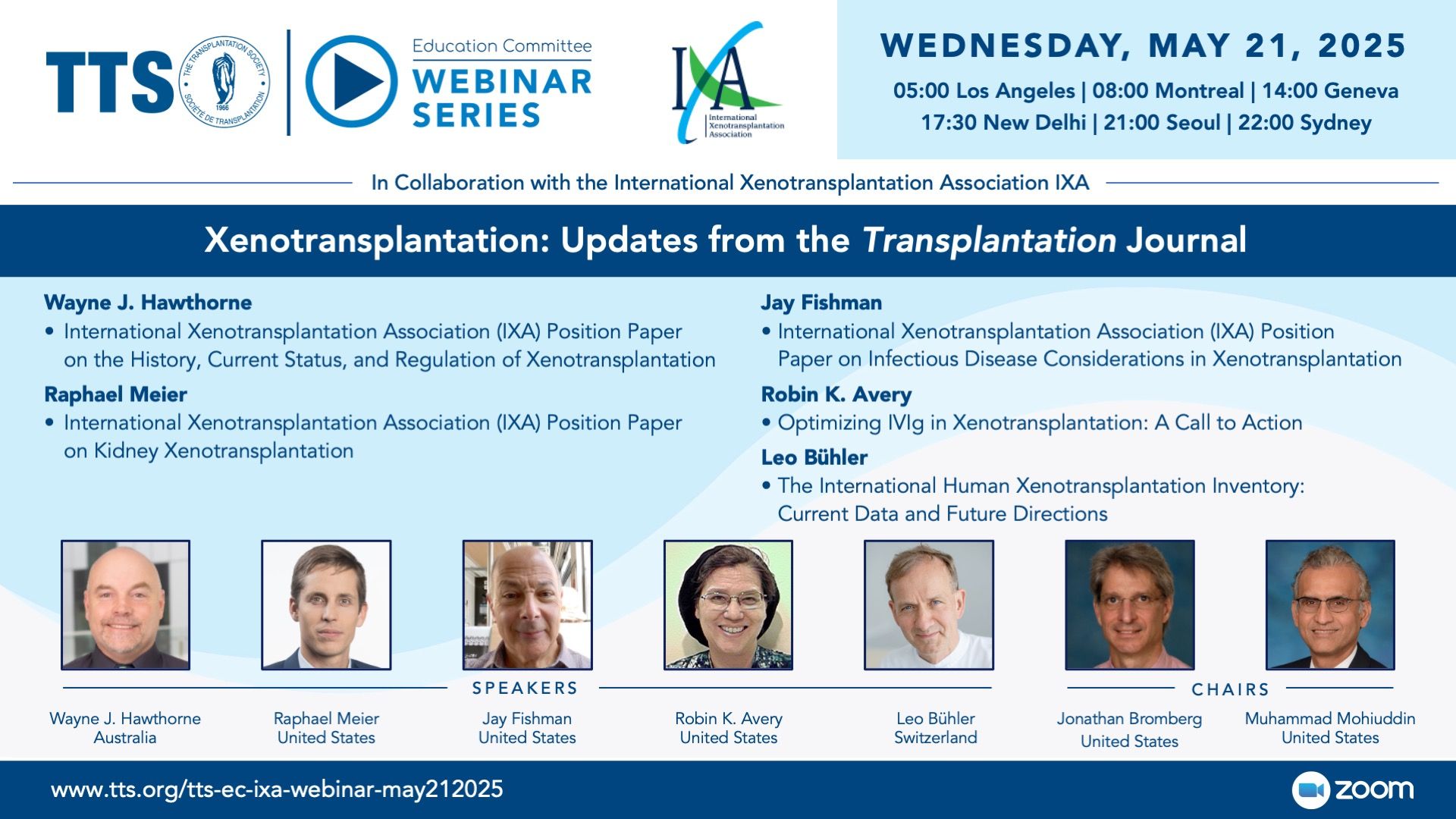
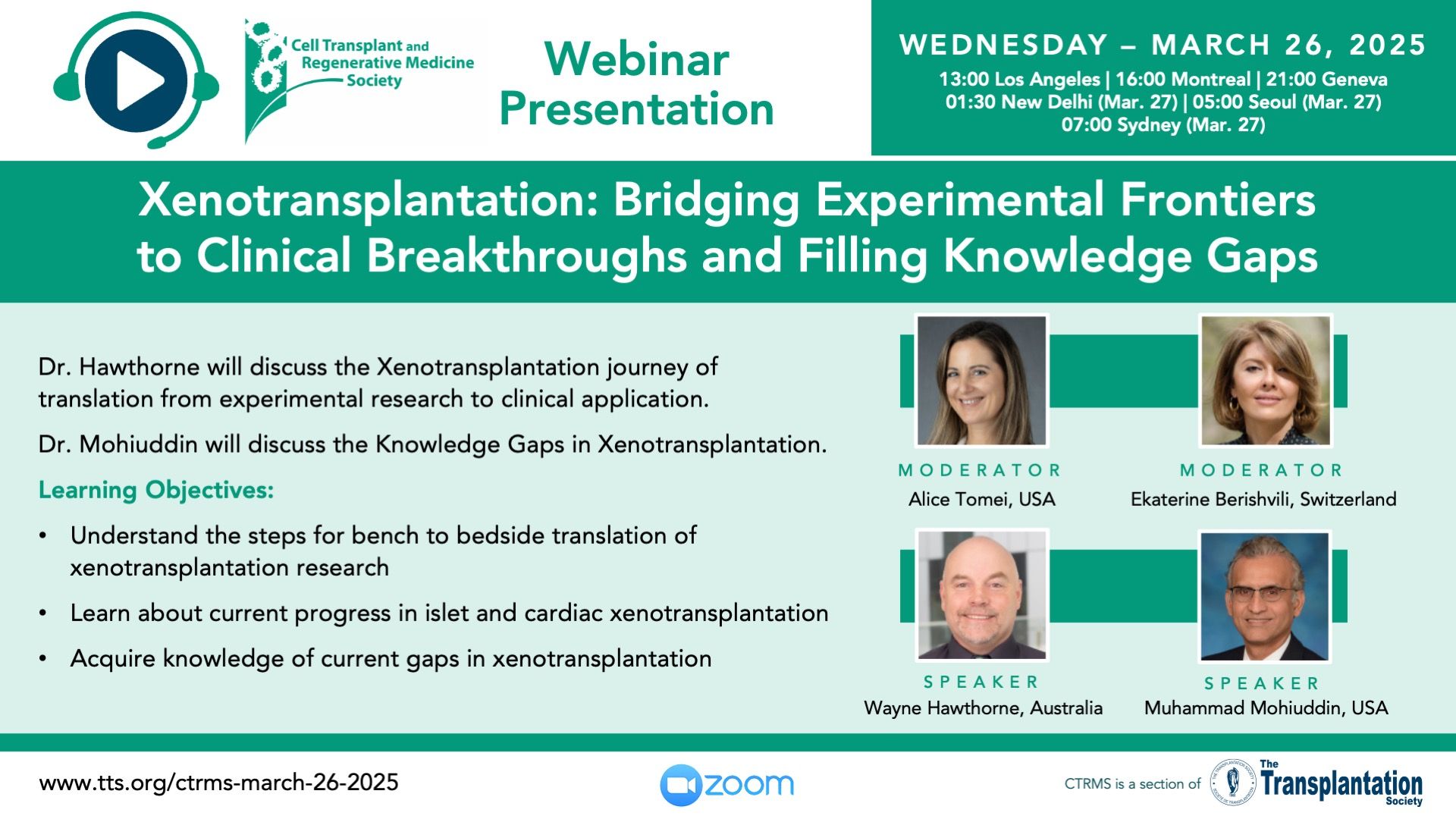
Recent Xenotransplantation Presentations
Council Message - April 2024
The recipient was a 62-year-old man living with end-stage kidney disease (ESKD) with a failed prior allograft and without functioning vascular access who was subsequently discharged from the hospital for continued care. Details are being prepared for peer-reviewed publication. This was a major milestone in advancing clinical xenotransplantation. This transplant was based on decades of preclinical xenotransplantation research at MGH and other xenotransplant research programs worldwide. The gene-edited pig kidney carried 69 genomic edits including multiple modifications to address immune and coagulation incompatibilities as well as inactivation of the porcine endogenous retrovirus (performed by eGenesis of Cambridge, Massachusetts).
A subsequent living xeno-kidney recipient at the NYU Langone Medical Center (NYU) in New York City, USA was for a 54 year old woman with heart and kidney failure who received a both a ventricular support device and subsequent gene edited combined thymo-kidney xenograft and is doing well. The added thymic graft is intended to enhance long term immune compatibility in the recipient. This compassionate use living xenotransplantation follows on the heels of studies at NYU of multiple xeno-kidney implants into brain dead human recipients.
Both are major steps in the quest to provide more readily available organs to patients. It is notable that the two teams have deployed differing immunosuppressive regimens and pigs carrying different genetic edits to achieve renal replacement. The field of transplantation will benefit from insights gained from this experience. These efforts depend on large teams at each center as well as important collaborations with industrial partners who have developed source animals, immunosuppressive agents, and diagnostic tools deployed for these therapies.
The field of xenotransplantation is most grateful to the two remarkable kidney recipients in these pioneering surgeries, whose courage and support have allowed these advances.
On behalf of the International Xenotransplantation Association Council
BOSTON – MARCH 2024 - Massachusetts General Hospital (MGH), a founding member of the Mass General Brigham health care system, announced the world’s first successful transplant of a genetically-edited pig (porcine) kidney into a 62-year-old man living with end-stage kidney disease (ESKD). Surgeons from the Mass General Transplant Center conducted the four-hour- long surgery on Saturday, March 16. The procedure marks a major milestone in the quest to provide more readily available organs to patients.
The patient is recovering well at MGH and is expected to be discharged soon.
Under the leadership of Leonardo V. Riella, MD, PhD, Medical Director for Kidney Transplantation, Tatsuo Kawai, MD, PhD, Director of the Legorreta Center for Clinical Transplant Tolerance, along with Nahel Elias, MD, Interim Chief of Transplant Surgery and Surgical Director for Kidney Transplantation, a genetically-edited pig kidney with 69 genomic edits was successfully transplanted into a living patient. MGH transplant clinicians and surgeons have nearly 30 years of experience with xenotransplantation research. IXA members in attendance were recognized at the press briefing including Drs. David Sachs, David Cooper, Richard (Robin) Pierson, Joren Madsen, and Jay Fishman.
“Mass General Brigham researchers and clinicians are constantly pushing the boundaries of science to transform medicine and solve significant health issues facing our patients in their daily lives,” said Anne Klibanski, MD, President and CEO, Mass General Brigham. “Nearly seven decades after the first successful kidney transplant, our clinicians have once again demonstrated our commitment to provide innovative treatments and help ease the burden of disease for our patients and others around the world.”
“The tireless commitment of our clinicians, researchers and scientists to improving the lives of our transplant patients – both current and future – is at the very heart and soul of academic medicine and what it means to work and provide care at Mass General Brigham,” said David F. M. Brown, MD, President, Academic Medical Centers, Mass General Brigham. “We are so thankful to the incredible staff throughout our hospitals who helped make this surgery a success, and to the patient for his bravery and courage.”
“The success of this transplant is the culmination of efforts by thousands of scientists and physicians over several decades. We are privileged to have played a significant role in this milestone. Our hope is that this transplant approach will offer a lifeline to millions of patients worldwide who are suffering from kidney failure,” Kawai said.
The pig kidney was provided by eGenesis of Cambridge, Mass., from a pig donor that was genetically-edited using CRISPR-Cas9 technology to remove harmful pig genes and add certain human genes to improve its compatibility with humans. Additionally, eGenesis scientists inactivated porcine endogenous retroviruses in the pig donor to eliminate risks due to these organisms in humans. Dr. Jay Fishman, President-elect of IXA, Professor of Medicine, and Director of the MGH Transplant Infectious Disease Program, with eGenesis scientists have developed an extensive testing strategy for the pigs and for the kidney recipient to reduce potential risks of infection. The procedure was performed under a single FDA Expanded Access Protocol (EAP) which was rigorously reviewed by the FDA before its approval in late February. This compassionate use protocol also included infusion of novel immunosuppressant drugs, tegoprubart, provided by Eledon Pharmaceuticals, Inc., and ravulizumab, provided by Alexion Pharmaceuticals, Inc.
“We are grateful for the courageous contribution of the patient and to the advancement of transplantation science,” said Mike Curtis, Chief Executive Officer, eGenesis. “We congratulate our collaborators at MGH on this historic milestone. We also recognize the work and dedication of the eGenesis team that made this achievement possible. This represents a new frontier in medicine and demonstrates the potential of genome engineering to change the lives of millions of patients globally suffering from kidney failure.”
This successful procedure in a living kidney recipient is a further historic milestone in xenotransplantation and extends observations in decedent recipients and recent cardiac xenotransplants to clinical applications. Kidneys are the most common organ needed for transplant with end-stage kidney disease rates estimated to increase 29-68 percent in the U.S. by 2030.
“The real hero today is the patient, as the success of this pioneering surgery, once deemed unimaginable, would not have been possible without his courage and willingness to embark on a journey into uncharted medical territory said Joren C. Madsen, MD, DPhil, Director of the MGH Transplant Center.
“The continued success of this groundbreaking kidney transplant represents a true milestone in the field of transplantation. It also represents a potential breakthrough in solving one of the more intractable problems in our field, that being unequal access for ethnic minority patients to the opportunity for kidney transplants due to the extreme donor organ shortage and other system-based barriers. This health disparity has been the target of many national policy initiatives for over 30 years, with only limited success. An abundant supply of organs resulting from this technological advance may go far to finally achieve health equity and offer the best solution to kidney failure – a well-functioning kidney – to all patients in need.” Williams said
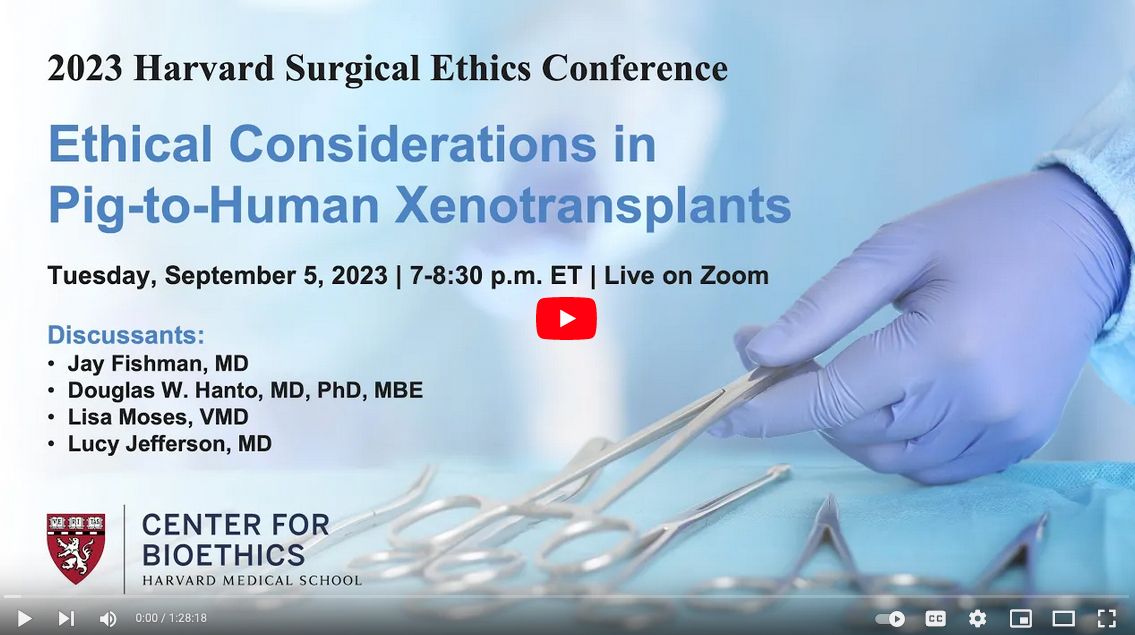
Ethical Considerations in Pig-to-Human Xenotransplantation
Thank You for Attending!

Thank you to our delegates, sponsors and exhibitors for making IXA 2025 a success!
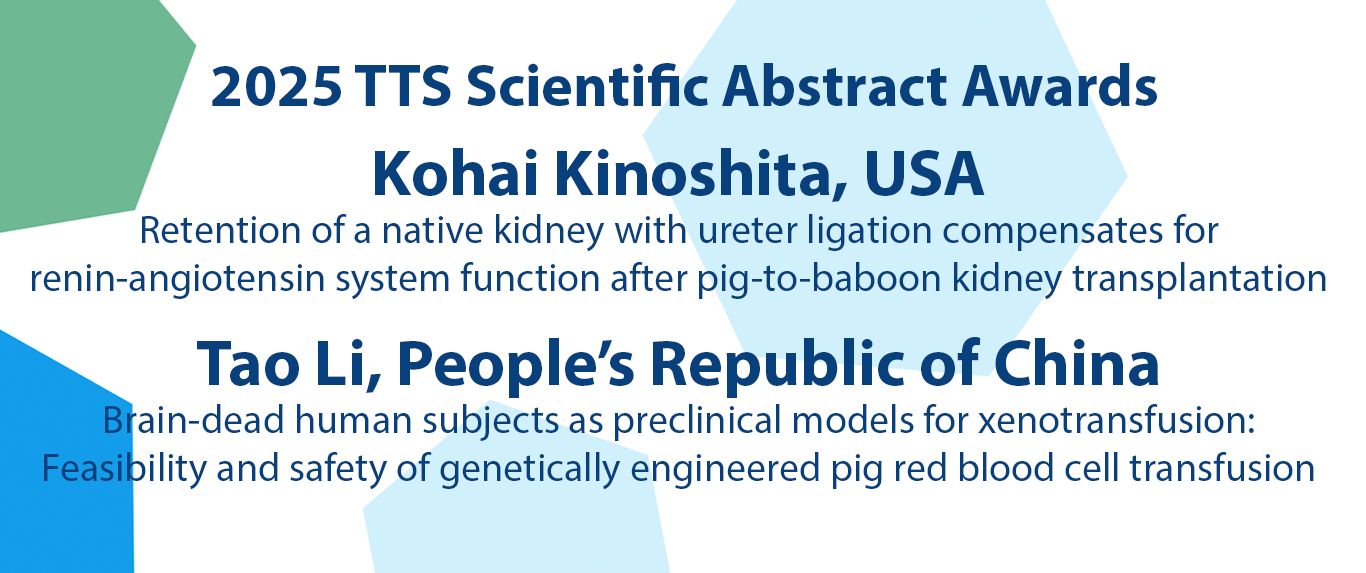
Congratulations to our 2025 Congress Award Winners
Social
Address
International Xenotransplantation Association
C/O The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada