2017 - IPITA
This page contains exclusive content for the member of the following sections: TTS, IPITA. Log in to view.
Islet Engraftment & Extra-hepatic Islet Implantation Sites
33.2 - Peri-islet vessels formation around human islets by stimulating intra-islet endothelial cells: Bioengineering approaches to improve islet survival after transplantation
Presenter: GOPAL, Loganathan, Louisville, United States
Authors: Gopalakrishnan Loganathan, Venkat M. Ramakrishnan, Brian Gettler, Leigh Kleinert, Siddharth Narayanan, Michael G. Hughes, James B. Hoying, Stuart K. Williams, Appakalai N. Balamurugan
Peri-islet vessels formation around human islets by stimulating intra-islet endothelial cells: Bioengineering approaches to improve islet survival after transplantation
G. Loganathan1, V. Ramakrishnan1, B. Gettler1, L. Kleinert1, S. Narayanan1, M. Hughes1, J. Hoying1, S. Williams1, A. Balamurugan1.
1Clinical Islet Cell Laboratory, Cardiovascular Innovation Institute, Department of Surgery, University of Louisville School of Medicine, Louisville, KY, USA,
Background: Native islets in the pancreas have a rich vascular supply however, the current islet isolation techniques completely severs the vasculature to islets. As such, these islets become avascular and many of the inner core cells die immediately after islet transplantation. Stimulation of intra-islet endothelial cells (IIEC) to form peri-islet capillaries (PIV) in vitro prior to transplantation may accelerate post-implant islet revascularization. In this study, we accelerated the formation of PIV to human islets (Bioengineered islets –BEI) by stimulating the IIEC with the addition of endothelial cell growth supplement (ECGS -a mixture of endothelial growth factors) in a 3D-culture system.
Methods and Materials: Human islets cells were isolated from donor research pancreases (n=15) and the islets were cultured in a 3D system using collagen-I gel with ECGS containing medium for 14 days. 2D cultured islets and 3D cultured islets without ECGS were used us control groups. Time-lapse microscopy (Cytation™ 5 with Augmented Microscopy™) was used to monitor islet-derived PIV growth every day for 14 days. CD31 and UEA-1 staining was used to confirm endothelial cells. Islet cell viability, basal and glucose stimulated insulin release tests were done after PIV formation To study diabetes reversal, the BEI were implanted in to diabetic nude mice at the subcutaneous site.
Results: Our approach induced cellular sprouting in human islets and were positive for endothelial cells (UEA-1) staining. The sprout formation was evident within 3 days of culture. The BEI maintained >90% cell viability, high insulin secretory capacity when compared to control islets and importantly, BEI reversed diabetes in mice model in subcutaneous site and histology image showed presence of blood vessels around the islets. Naked islets cultured in a 2D environment did not form any sprout. However, ECGS medium maintained intra-islet viability when compared to standard CMRL-1066 culture medium. The presence of ECGS and 3D-culture system induced cellular sprouting from human islets within 7 days. 3D-cultured islets maintained >90% cellular viability after 14 days, whereas 2D-cultured islets displayed disintegration of their borders as well as reduced viability. Statistically significant difference (P<0.05) was observed in number of sprouts when islets were cultured in 3D environment without ECGS.
Conclusions: We demonstrated that 3D matrix support and ECGS are required to elicit successful, spontaneous PIV formation in human islets. The standard human islet culture medium (CMRL-1066) and techniques (2D – free-floating islets) did not maintain the viability of intra-islet endothelial cells. ECGS medium may necessary to maintain intra-islet endothelial cell viability. ECGS with 3D culture may provide unique avenues to accelerate islet neovascularization following transplantation.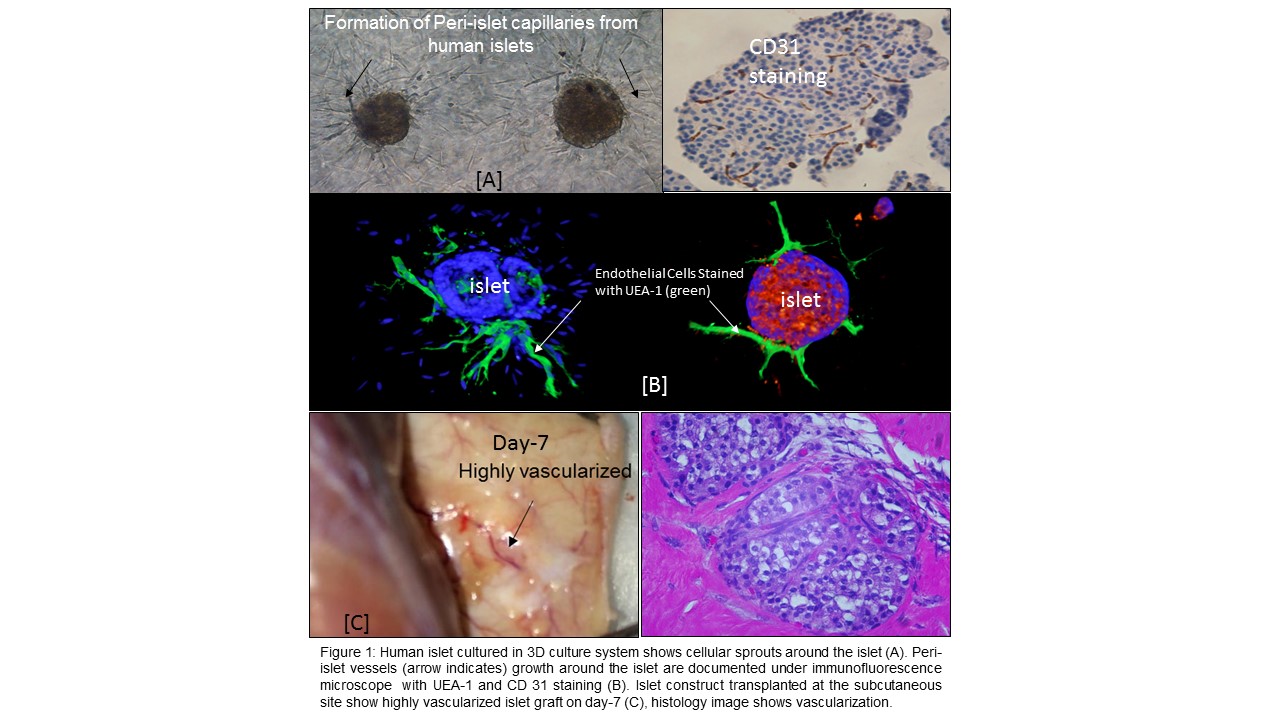
Important Disclaimer
By viewing the material on this site you understand and accept that:
- The opinions and statements expressed on this site reflect the views of the author or authors and do not necessarily reflect those of The Transplantation Society and/or its Sections.
- The hosting of material on The Transplantation Society site does not signify endorsement of this material by The Transplantation Society and/or its Sections.
- The material is solely for educational purposes for qualified health care professionals.
- The Transplantation Society and/or its Sections are not liable for any decision made or action taken based on the information contained in the material on this site.
- The information cannot be used as a substitute for professional care.
- The information does not represent a standard of care.
- No physician-patient relationship is being established.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
