2017 - IPITA
This page contains exclusive content for the member of the following sections: TTS, IPITA. Log in to view.
Poster Viewing
9.27 - Large-Scale and Sorting of Encapsulated Human Islet Preparation into Resolved Empty from Loaded Subpopulations: Proof-Of-Concept
Presenter: JE, Mendoza-Elias, Chicago, United States
Authors: Joshua Mendoza-Elias, Enza Marchese, Yuan Xian, Ling-jia Wang, James McGarigle, Sofia Ghani, Doug Isa, Chun-Chieh Yeh, José Oberholzer, Yong Wang
Large-Scale and Sorting of Encapsulated Human Islet Preparation into Resolved Empty from Loaded Subpopulations: Proof-Of-Concept
J. Mendoza-Elias1,2, E. Marchese1, Y. Xian1,2, L. Wang1, J. McGarigle1, S. Ghani1, D. Isa1, C. Yeh3, J. Oberholzer1, Y. Wang1,2.
1Department of Surgery/Transplant, University of Illinois at Chicago, Chicago, USA, ; 2Department of Bioengineering, University of Illinois at Chicago, Chicago, USA, ; 3Department of Surgery, China Medical University Hospital, Taichung, Taiwan,
Encapsulation is a promising approach to overcome the need for immunosuppression by protecting islet tissues from host immune rejection. The most commonly investigated method for islet encapsulation therapy is alginate microencapsulation, which has been shown to ensure efficient nutrient influx, insulin release, and waste exchange. However, due to the nature of the encapsulation process the production of empty capsules (microcapsules without islets) is an unavoidable by-product creating several obstacles. First, studies have demonstrated that empty alginate microspheres may potentiate foreign-body reactions that may result in encapsulated islet graft rejection. Second, empty microcapsules significantly contribute and increase the encapsulated islet preparation implanted into the animal model or potential patient. Third, this may limit transplant sites and implantable islet mass. Consequently, empty capsule by-products are a technical limitation in the clinical application of islet microencapsulation. In general, manual removal of the empty capsules post encapsulation is challenging and unpractical due to the large capsule population and potential contamination. Other methods have had limited success; but, are difficult to scale-up to the industrial scale and speed required by the clinic.
In this study, we demonstrate that a continuous density gradient can sort an encapsulated human islet preparation into resolved empty and loaded capsules subpopulations. We demonstrated the sorting of 10,000 islet equivalents (IEq) and 20,000 IEq human islet preparations encapsulated in a 500 µm alginate calcium and barium capsule at a seeding density of 10,000 IEq/mL combined with equal volume of empty alginate. Furthermore, we characterize pre-sorted (PRE) and post-sorted (POST) encapsulated islet preparations and characterize: preparation volume reduction, islet mass loss, loaded-capsule-percent-composition, morphology, viability scores, and function as evaluated by: glucose induced insulin secretion assay, intracellular calcium flux assay, and mitochondrial potential changes.
In summary, for sorting 20,000 IEq preparation, we found the process reduced encapsulated islet preparation volume (-33.33% ± 2.95) with a non-significant loss in IEq (PRE vs. POST: 18,239.3 ± 952.20 vs. 16,156.15 ± 4,772.66, p=0.4996); and enrichment in loaded-capsule-percent-composition (PRE vs. POST: 27.11% ± 5.79 vs 59.18% ± 9.85, p=0.0177). Furthermore, islet viability and function was not significantly adversely impacted and the observation that increasing encapsulated islet density correlates with: (i.) increasing islet viability scores (PRE vs. POST: 65.33% ± 4.55 vs. 77.63% ± 2.35, p=0.0085); (ii.) GSIS bulk insulin released during 25 mM glucose challenge (20.58 ± 2.83 µUI/10 islets vs. 52.40 ± 7.55 µUI/10 islets, p=0.0169); (iii.) GSIS stimulation index (PRE vs. POST: 0.7069 ±0.05 vs. 1.51 ± 0.20, p=0.00185); (iv.) AUC of intracellular calcium influx under 25 mM glucose stimulation (PRE vs. POST: 1993.76 ± 13.56 vs. 2178.16 ± 8.33, p<0.0001); (v.) intracellular calcium influx under 30 mM KCL stimulation (PRE vs. POST: 1638.22 ± 22.39 vs. 1714.22 ± 16.97, p<0.0067); while not significantly impact metabolic function as indicated by non-significant changes in mitochondrial potential changes.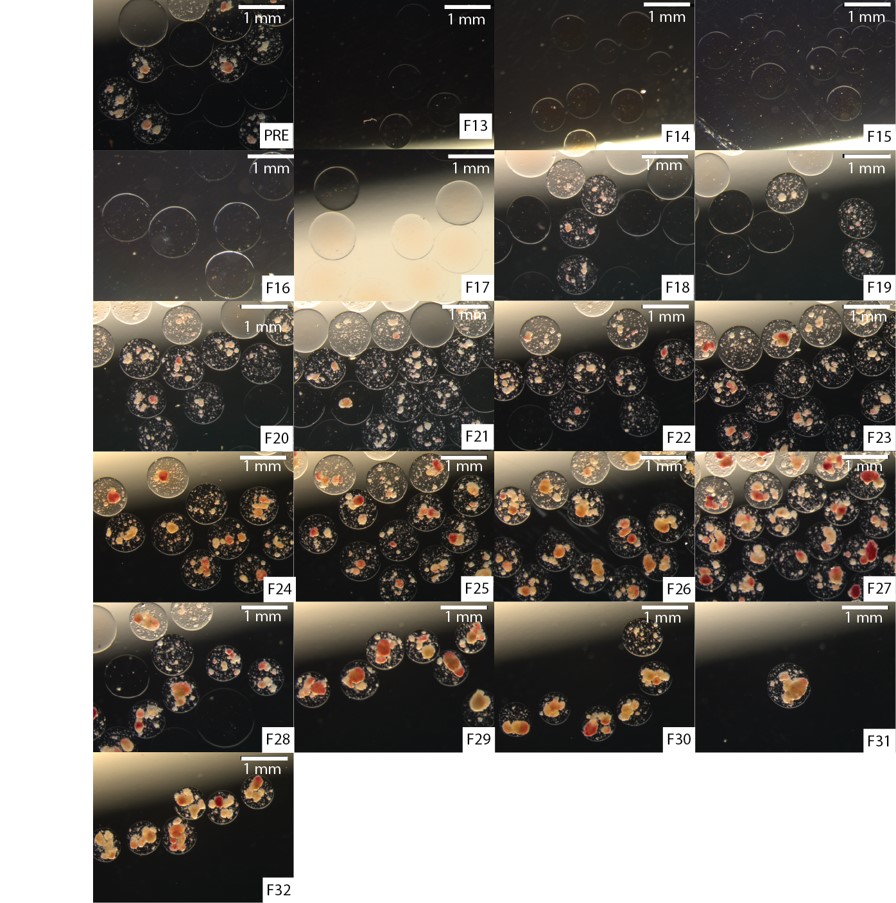
Important Disclaimer
By viewing the material on this site you understand and accept that:
- The opinions and statements expressed on this site reflect the views of the author or authors and do not necessarily reflect those of The Transplantation Society and/or its Sections.
- The hosting of material on The Transplantation Society site does not signify endorsement of this material by The Transplantation Society and/or its Sections.
- The material is solely for educational purposes for qualified health care professionals.
- The Transplantation Society and/or its Sections are not liable for any decision made or action taken based on the information contained in the material on this site.
- The information cannot be used as a substitute for professional care.
- The information does not represent a standard of care.
- No physician-patient relationship is being established.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
