2011 - 10th Meeting - IHCTAS
This page contains exclusive content for the member of the following sections: TTS, IHCTAS. Log in to view.
Posters
2.19 - INDUCTION OF DONOR-SPECIFIC TOLERANCE TO A COMPOSITE ISLET GRAFT VIA LOCAL OVER-EXPRESSION OF INDOLEAMINE 2,3 DIOXYGENASE
Presenter: Reza Baradar, Jalili, Vancouver, BC, Canada
Authors: Reza Baradar Jalili, Azadeh Hosseini-Tabatabaei, Ryan Hartwell, Garth Warnock, Aziz Ghahary
INDUCTION OF DONOR-SPECIFIC TOLERANCE TO A COMPOSITE ISLET GRAFT VIA LOCAL OVER-EXPRESSION OF INDOLEAMINE 2,3 DIOXYGENASE
Reza Baradar Jalili1, Azadeh Hosseini-Tabatabaei1, Ryan Hartwell1, Garth Warnock1, Aziz Ghahary1.
1Department of Surgery, University of British Columbia, BC, Canada.
Transplantation of islets of Langerhans represents a viable treatment for type 1 diabetes. However, requirement of systemic immunosuppression post-transplant is a major drawback to clinical islet transplantation. Indoleamine 2,3 dioxygenase (IDO) is a cytosolic enzyme that catabolizes the essential amino acid tryptophan into kynurenine. IDO expression generates a microenvironments low in tryptophan and high in kynurenines within which activated T-cells can not proliferate and are eliminated through apoptosis. Here we report that local expression of IDO in a composite islet graft induces donor-specific immune tolerance and significantly prolongs graft survival.
We engineered composite islet grafts by embedding Balb/c mouse islets within genetically modified IDO over-expressing fibroblast populated collagen matrices. These grafts were then transplanted into renal subcapsular space of STZ-induced diabetic C57Bl/6 (B6) mice. No systemic immunosuppressive agent was administered to graft recipient mice.
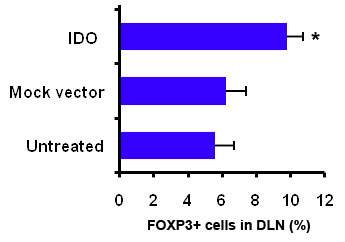
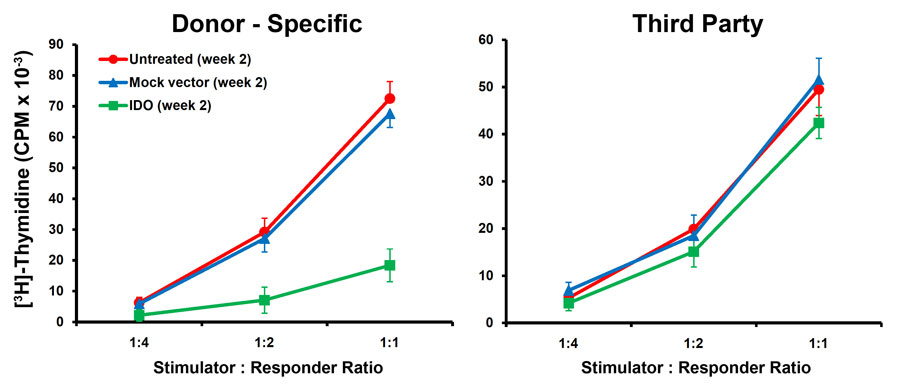
IDO-expressing islet grafts survived significantly longer than control grafts (41.2 ± 1.64 days vs. 12.9 ± 0.73 days). Over-expression of IDO significantly increased the number of FOXP3+ cells in graft draining lymph nodes of IDO composite recipient animals (Fig 1). These cells efficiently suppressed in vitro proliferation of lymphocytes from donor (B6) but not from a third party (C3H) mouse strain (Fig 2). Local IDO expression also resulted in significantly lower levels of intra-graft pro-inflammatory cytokines (IFN-g, IL-2, and IL-17) and chemokines (CXCL9 and CXCL10). On the contrary, intra-graft levels of anti-inflamatory cytokines (IL-4 and IL-10) were significantly higher in the IDO group. Moreover, a significant delay in anti-donor alloantibodies production as well as a TH2 antibody subclass switching (IgG1 vs. IgG2c) was found in IDO grafts recipient animals.
These findings show that local over-expression of IDO can induce donor-specific tolerance via generation of FOXP3+ cell and a TH2 anti-inflammatory immune response. Findings of this study set the stage for development of a non-rejectable islet graft using local expression of IDO.
Important Disclaimer
By viewing the material on this site you understand and accept that:
- The opinions and statements expressed on this site reflect the views of the author or authors and do not necessarily reflect those of The Transplantation Society and/or its Sections.
- The hosting of material on The Transplantation Society site does not signify endorsement of this material by The Transplantation Society and/or its Sections.
- The material is solely for educational purposes for qualified health care professionals.
- The Transplantation Society and/or its Sections are not liable for any decision made or action taken based on the information contained in the material on this site.
- The information cannot be used as a substitute for professional care.
- The information does not represent a standard of care.
- No physician-patient relationship is being established.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
