TID Issues COVID Guidance Update (August 20)

Read the latest Transplantation - Vol. 105, No. 9, September 2021
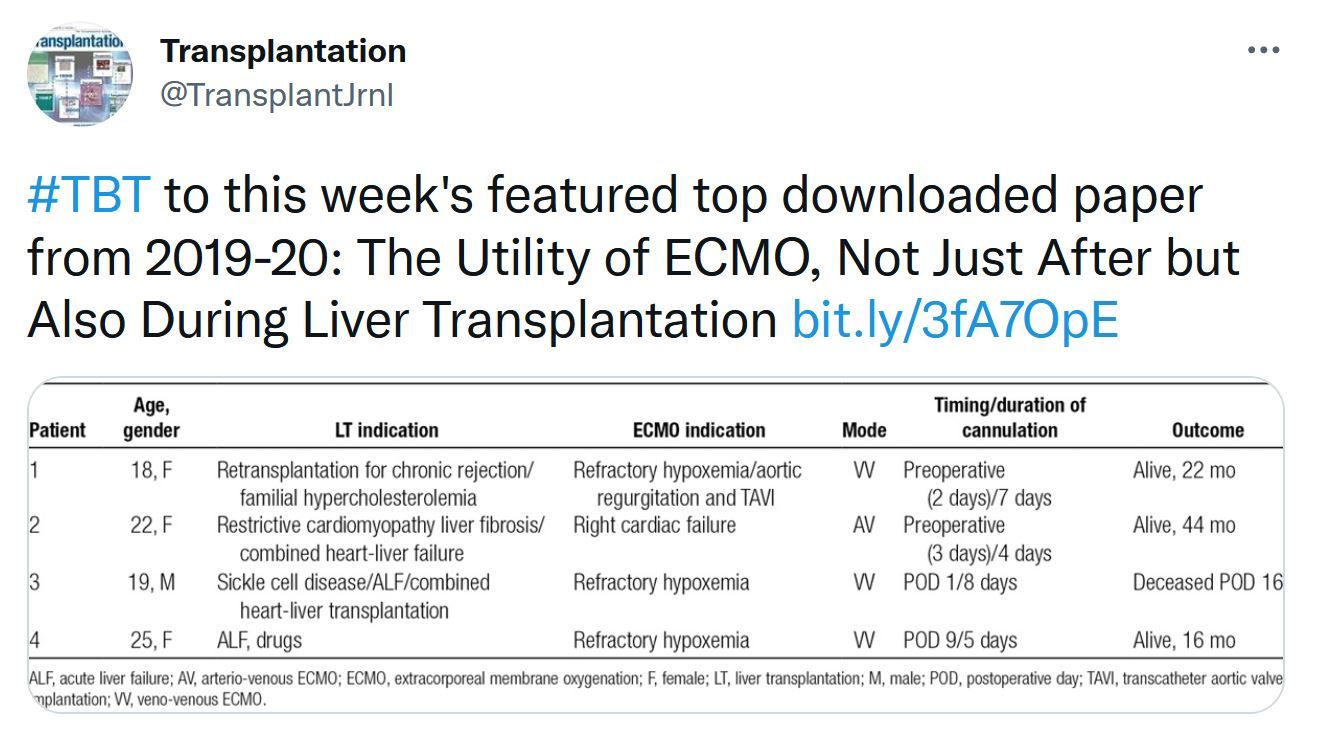
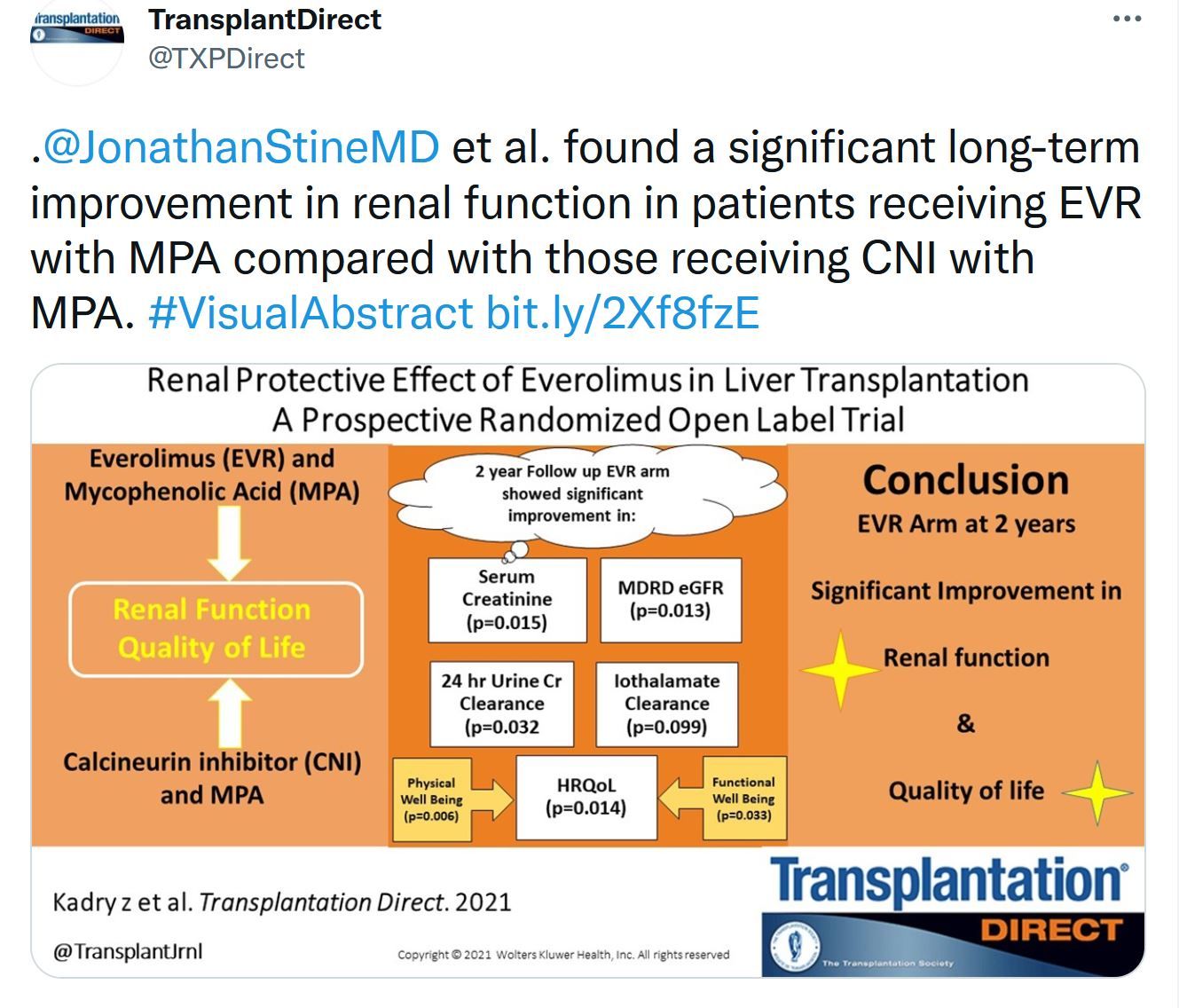
This issue includes data and analysis of the problem of COVID vaccination in the transplant population. Important data that will probably only be superseded by controlled clinical trials. The Notify project's first report on donor-derived disease. Liver transplantation data is focused on donor selection the early posttransplant period – extubation. The role of machine learning in donated kidney discard adds to the options to be considered to both improve our selection and increase utilization of the donor potential.
IPTA 2022: Approaching Abstract Deadline
The PEDIATRIC transplant community is meeting IN PERSON in Prague!
Mark March 26-29, 2022 in your agenda.

View a special message from Dr. Carlos O. Esquival – IPTA President

WIT - Picture-a-Scientist Networking Session
TTS will be exhibiting at ESOT in Milan!
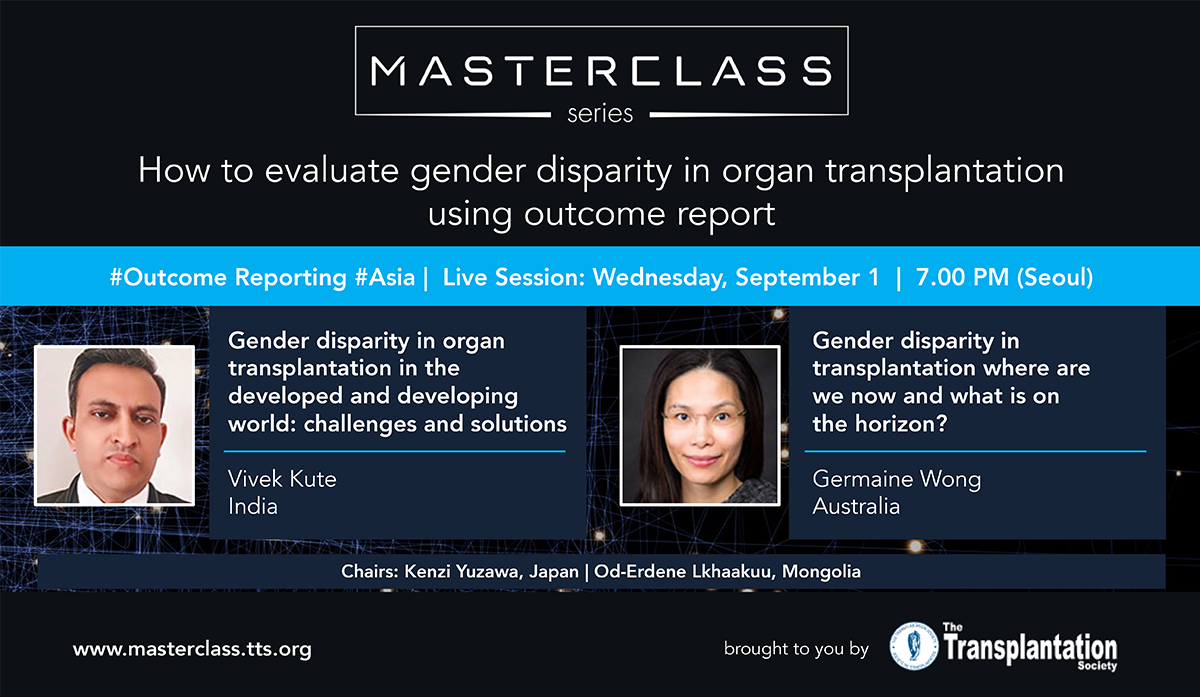
TTS Masterclasses: Outcome Reporting Series Continues
TTS Members can now register for FREE for the last two Masterclass Series on Transplant Immunology and Outcome Reporting. Members must pre-register to attend the live Masterclasses.

TTS is pleased to announce that the remaining two Masterclass Series (Transplant Immunology Series & Outcome Reporting Series) have been granted European CME credits (ECMEC®s) by the European Accreditation Council for Continuing Medical Education (EACCME®).
Attendance at the live Masterclasses is mandatory to receive the certificate.
Upcoming Masterclasses
In case you missed it ... SAT Launches Online Training Course
Latest Video Posted
August 18, 2021
09:00 (-4 GMT - Montreal time)
View the discussion on solid organ transplant recipient with post-transplant central
nervous system (CNS) infection. Our panel will discussed the differential diagnoses
and the diagnostic approaches including novel microbiologic tests. Click button below for details.
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!