Announcing the TTS 2022 “Living Legends” Award Recipients
In recognition of members who have contributed significantly to the field, this year TTS is recognizing as “Living Legends” members with extraordinary reputations that have contributed tremendously to the field either through research, innovation or mentorship.
International
Latin America
Argentina
Transplantation Updates

Transplantation - September Issue
Transplantation - Week's Most Downloaded Paper
Moving Toward Transplant Tolerance: Is Targeting Donor Antigen-presenting Cells the Key?
Donor-specific tolerance is a long-held, elusive goal for transplantation. While robust dominant tolerance across major histocompatibility complex (MHC) barriers has been achieved by numerous manipulations in rodents, translation into nonhuman primate (NHP) models or clinical practice has been challenging. Apart from the induction of hematopoietic chimerism there are very few examples of allograft tolerance in clinical practice. Whether persistent chimerism (versus that of a transient nature) is an absolute requirement for tolerance has been debated, however without resolution. Furthermore, the induction of chimerism requires extensive preconditioning and is associated with significant toxicity, particularly graft versus host disease, factors which have limited its clinical feasibility.Transplantation Direct - Highlighted Tweet
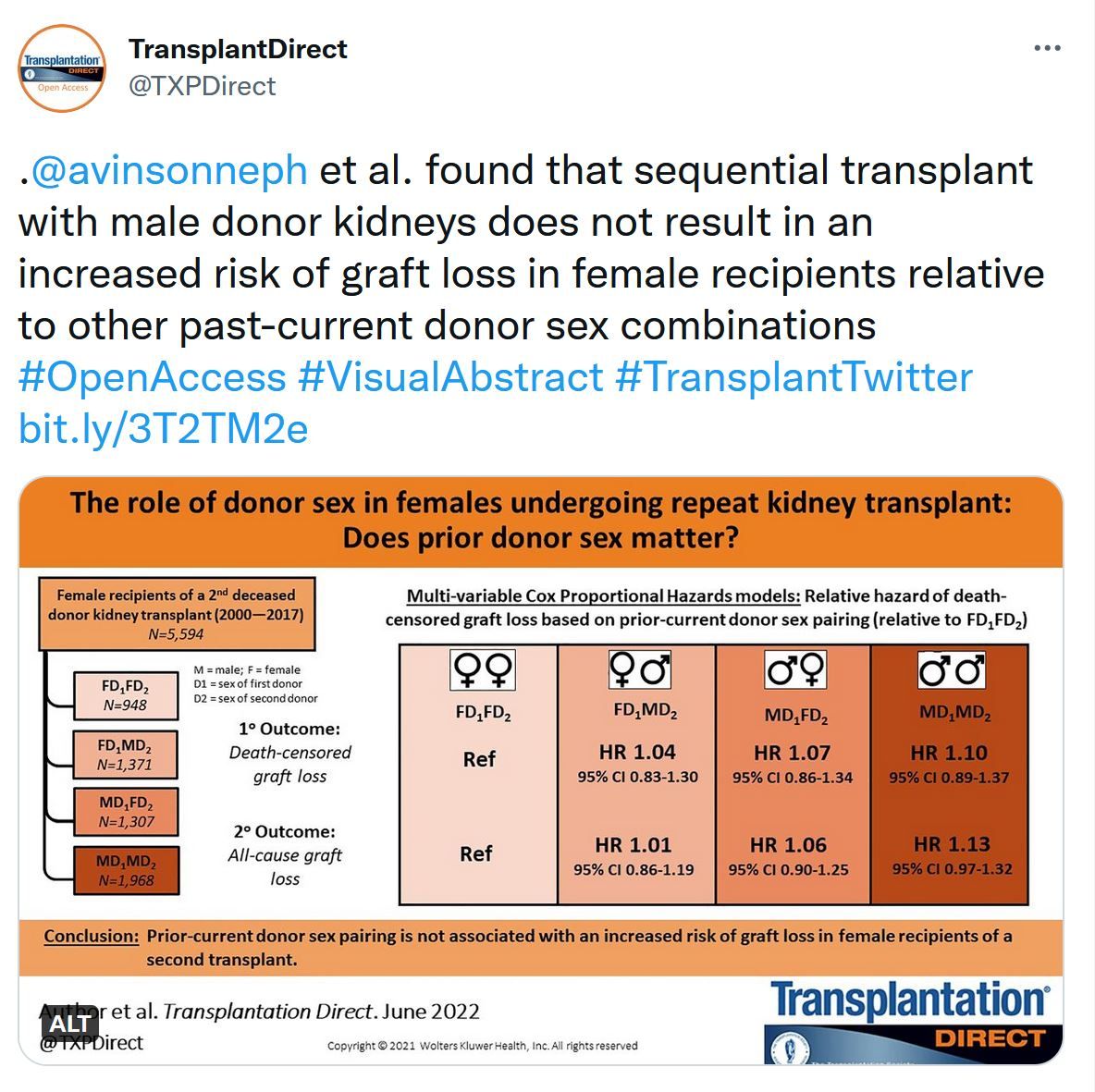
The Role of Donor Sex in Females Undergoing Repeat Kidney Transplant: Does Prior Donor Sex Matter?
Female recipients of male donor kidneys are at increased risk for graft failure because of the HY antigen effect. However, whether prior transplant with a male donor impacts subsequent transplant outcomes is unknown. Therefore, the purpose of this study was to determine whether prior male-current male donor sex is associated with an increased risk of graft failure in female recipients.TTC Public Workshop - September 22, 2022 @ 8AM ET
TTS Needs Assessment Survey
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!