Season’s Greetings and Happy Holidays!

This time of year, our thoughts turn gratefully to our family, friends and colleagues
who make our success possible. We thank you for all that you do,
and wish you all the best this holiday season and beyond.
Transplantation Direct - January 2023 Issue
The first issue of Transplantation Direct for 2023 ready for viewing!
One article examines the effect of multiple waves of COVID-19 on kidney transplant program adaptations in the US, and another reports the latest on humoral responses to vaccination in the Omicron era. Also in kidney transplantation, we have studies on immunosuppression management after allograft failure, Phaeohyphomycoses infectious complications, posttransplant cardiovascular disease, and use of Corline Heparin Conjugate during hypothermic machine perfusion in a Phase I clinical trial. On the topic of liver transplantation, there is an article analyzing the recent Acuity Circles allocation policy in the US and a case study on percutaneous direct puncture of a retropancreatic splenic vein and portal thrombectomy after living donor transplantation. In addition, we have articles on the value of kidney histology for predicting cardiorenal syndrome before heart transplantation. In islet transplantation, there is an innovative experimental study on using polycaprolactone scaffolds subcutaneously to allow repeated islet implantations. In support of transplantation research, the Australians have managed to integrate organ donation with sample collection to serve as a unique biobank; their strategy is described herein. We welcome everyone to visit our Transplantation Direct website for open access to all the details and look forward to serving you in 2023.Press Release - Critical Path Institute (C-Path)
C-Path’s Transplant Therapeutics Consortium Receives EMA Qualification Opinion for iBox Scoring System
2023 International Transplantation Science Meeting

The ITS 2023 committee is extending the abstract submission deadline to January 16, 2023 at 23:59 EST due to the general demand from the basic science community.
Transplantation Updates

Transplantation - Highlighted Articles
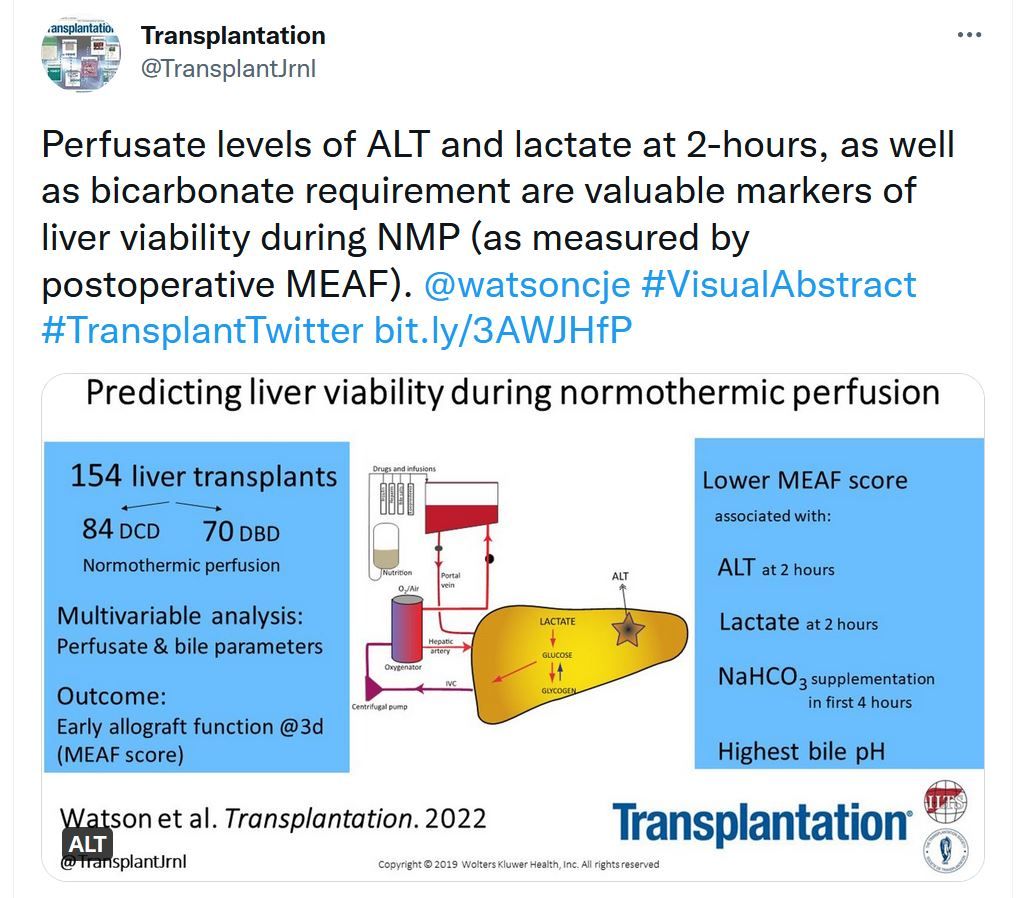
“Predicting Early Allograft Function After Normothermic Machine Perfusion
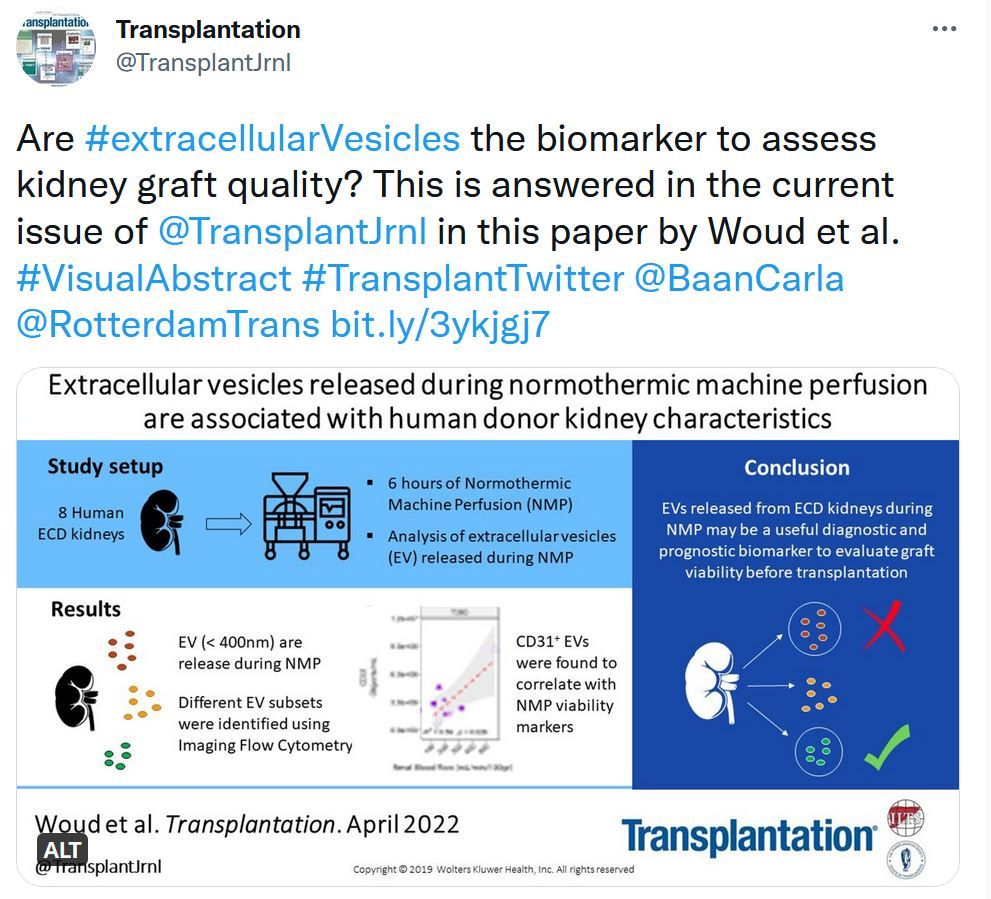
Normothermic ex situ liver perfusion is increasingly used to assess donor livers, but there remains a paucity of evidence regarding criteria upon which to base a viability assessment or criteria predicting early allograft function.Extracellular Vesicles Released During Normothermic Machine Perfusion Are Associated With Human Donor Kidney Characteristics
Extracellular vesicles (EVs) are tissue-specific particles released by cells containing valuable diagnostic information in the form of various biomolecules. The characterization of EVs released by kidney grafts during normothermic machine perfusion (NMP) may present a promising avenue to assess graft status before transplantation.Transplantation Direct - Highlighted Tweet
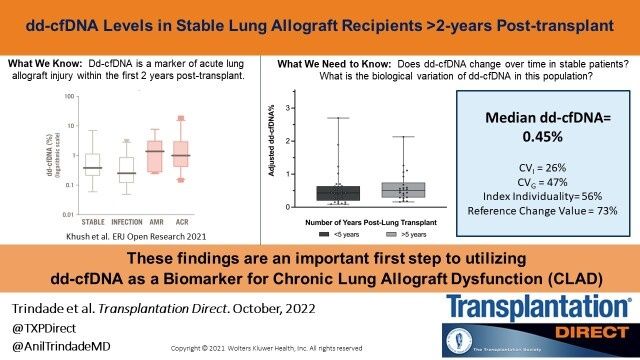
Assessment of dd-cfDNA Levels in Clinically Stable Lung Allograft Recipients Beyond the Initial 2 y Posttransplant
Donor-derived cell-free DNA (dd-cfDNA) is a useful biomarker for the diagnosis of acute allograft injury within the first 1 to 2 y after lung transplant, but its utility for diagnosing chronic lung allograft dysfunction (CLAD) has not yet been studied. Understanding baseline dd-cfDNA kinetics beyond the initial 2 y posttransplant is a necessary first step in determining the utility of dd-cfDNA as a CLAD biomarker. We seek to establish baseline dd-cfDNA% levels in clinically stable lung allograft recipients who are >2 y posttransplant.This week some section members were targeted by fraudsters impersonating (spoofing) other members, urgently requesting money on behalf on another colleague utilizing non-TTS e-mail addresses (in this case aol.com emails).
FBI - Difference between Spoofing and Phishing schemes
https://www.fbi.gov/how-we-can-help-you/safety-resources/scams-and-safety/common-scams-and-crimes/spoofing-and-phishing
How to Recognize and Avoid Phishing Scams (by the USA Office of Inspector General)
https://consumer.ftc.gov/articles/how-recognize-and-avoid-phishing-scams
Google - Avoid and report phishing emails
https://support.google.com/mail/answer/8253?hl=en
The scheme is very simple and basic in the approach
Step 1: The fraudster creates an email account with a large email provider (aol.com, gmail, outlook, yahoo, hotmail, etc). They then use the name of the person they are impersonating, or an acronym of a recognizable institution (for example president.ipita@aol.com) in the user portion of the email address.
Step 2: They visit a website and scrape the names in the council list or committee members list then search for their email addresses on PubMed of Google.
Step 3: They will, in most cases, send emails requesting emergency financial assistance (stuck an an airport, lost their wallet, etc) and they will ask for you to call them or send money by western union, moneygram, worldremit. wise, etc). The common denominator is the amounts are usually <$1000 however they can be larger but usually with bigger amounts they will ask for a wire transfer. Usually they will try to get you on the phone or simply send you an email with how to send the money.
Step 4: If they get someone on the phone there is an added danger if they get you to log into a website or click a link while on the phone. This spoofing attempt may turn into a Phishing attempt to gain control of your computer.
TTS-ILTS Paired Transplant Centers Program - Application Deadline: January 1
IPTA 2023 - Extended Early-Bird Registration Deadline
Latest Recordings
SPLIT is running a "4th Year Fellow Series" which includes talks on advanced hepatology and pediatric transplantation topics aimed to increase the knowledge of 4th year liver transplant fellows globally.
Survey - Using Social Media to Promote Cutting-Edge Research in Transplantation
Greetings,
Researchers from NYU Langone Health are conducting a research study titled Using Social Media to Promote Cutting-Edge Research in Transplantation. Dr. Macey Levan from the Departments of Surgery and Population Health at NYU Langone Health is the Principal Investigator leading this research. The purpose of this study is to learn how members of the Transplantation community use social media for professional purposes and how they see themselves using it in the future.
You qualify to complete this study if you fall under one of the following categories:
- Subscribers to the weekly email of new published articles in the Transplantation or Transplantation Direct journals
- Have submitted an article to the Transplantation or Transplantation Direct journals in the past 5 years
- Member of The Transplantation Society (TTS)
- Subscribers to newsletters from the TTS (Quarterly - Tribune, Weekly - Tribune Pulse)
Participation in this study is voluntary and will consist of completing an online survey about your thoughts on how you use social media for professional purposes and how you see yourself using it in the future. It will take about 5 minutes of your time.
If you are interested in participating, please click the link below to complete the survey:
https://nyumc.qualtrics.com/jfe/form/SV_bxvqr2xMkhhuyeW
If you have any questions or concerns about this study, please contact Dr. Levan at Macey.Levan@nyulangone.org.
This study has been reviewed by the NYU Langone Health Institutional Review Board (Study Number: 22-00681).
Sincerely,
The Editors and Communications
Offices of the Transplantation Journal
& Transplantation Society
ISODP December 2022 Journal Watch Just Released

To all the Journal Watch Readers for 2022,
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!