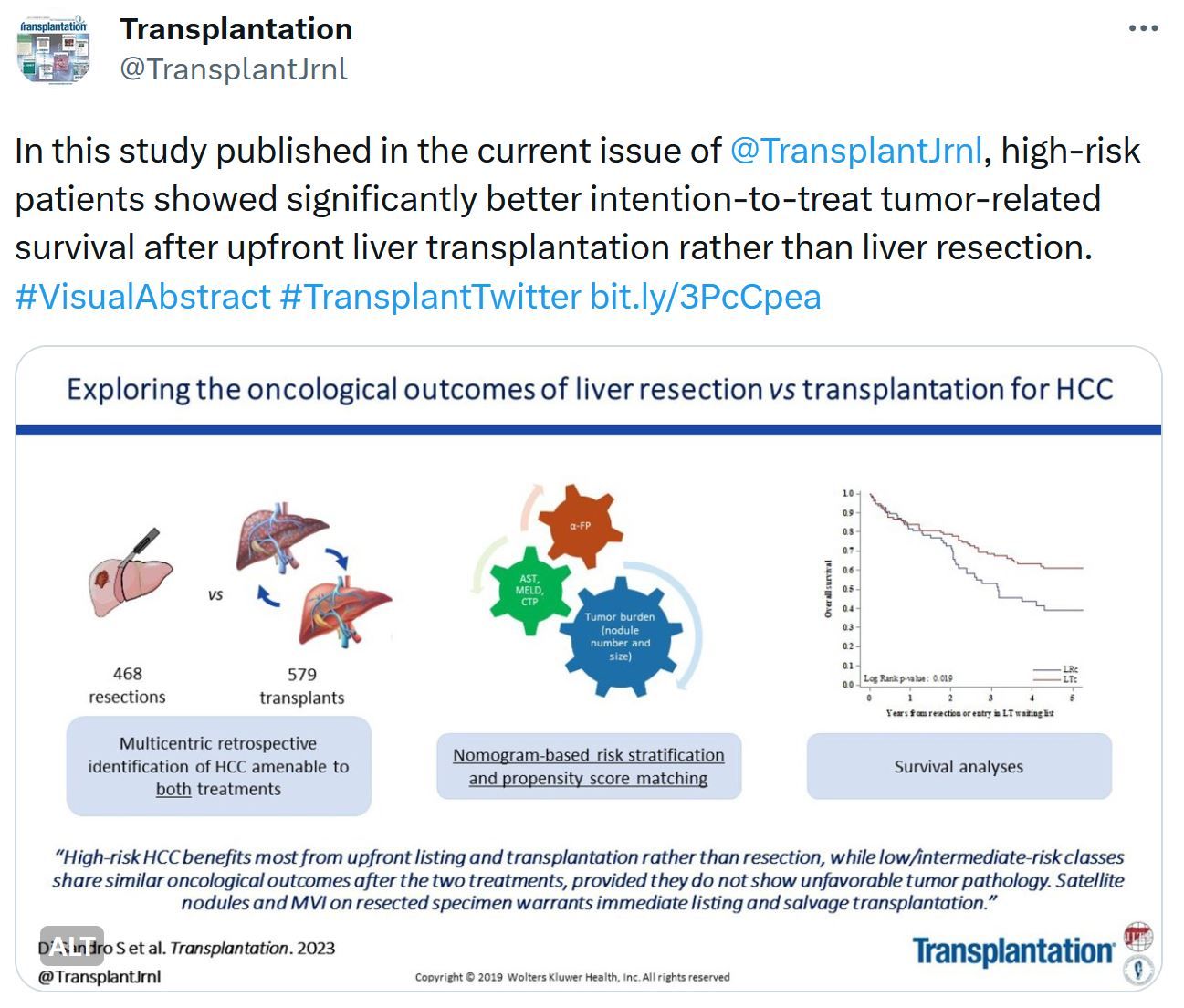
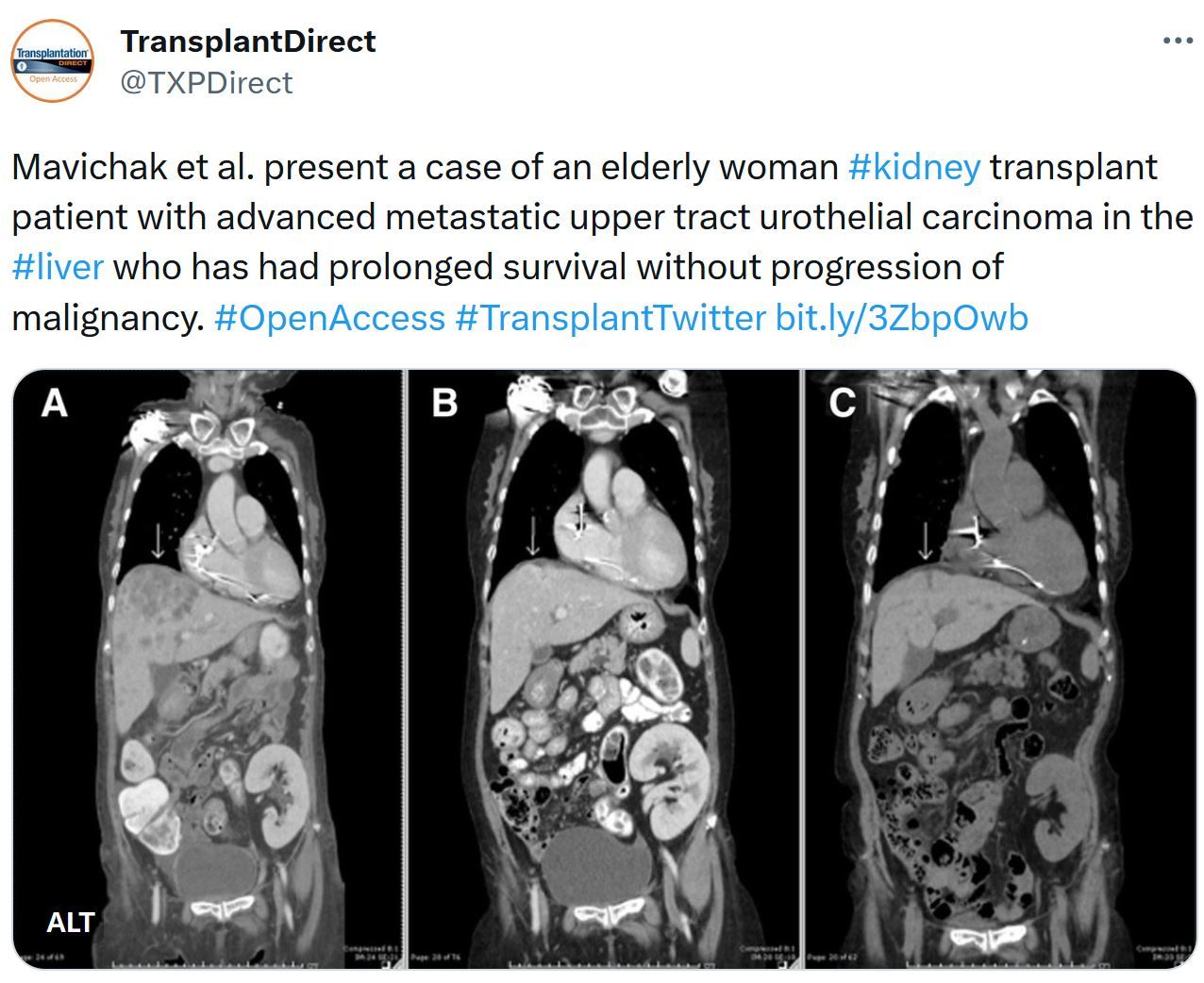
Just Released - Transplantation Journal - October Issue
This issue contains a number of important papers that you cannot afford to miss. First, the ILTS consensus guidelines created in collaboration with the iLDLT and LTSI societies provide guidelines for all aspects of Small for Size Syndrome. Second, biomarker sciences are well covered in an overview of Liver biomarkers, two brilliant articles on proteomics, one from a more technical and the other a more clinical perspective, an overview of RNA Seq approaches and an authoritative analysis of biomarkers for tolerance. Elsewhere in the issue are papers on bone mineral disorders after transplantation, liver allocation in children, frailty in lung recipients and much more.
Table of Contents
Around the World
Research Highlights
Game Changer
Special Feature
Meeting Report
Commentaries
- Organ Allocation in Children With Chronic Liver Disease: Quest for an Ideal Prognostic Model Continues!
- First Sips From the Holy Grail?
- Frailty: A Strong Impact on Lung Transplant Outcomes?
- Vaccine-preventable Disease After Transplantation: A Missed Opportunity
- The Need for Animal Research in the Field of Uterus Transplantation in Males
Reviews
- Current Status of Mineral and Bone Disorders in Transplant Recipients
- Novel Noninvasive Biomarkers in Liver Transplantation: A Tool on the Doorstep of Clinical Utilization
- Proteomics: Its Promise and Pitfalls in Shaping Precision Medicine in Solid Organ Transplantation
- Contribution of Proteomics in Transplantation: Identification of Injury and Rejection Markers
- Standardization and Interpretation of RNA-sequencing for Transplantation
Original Basic Science
- Transplantation of the Uterus in the Male Rat
- Modeling the Effects of IL-1β-mediated Inflammation During Ex Vivo Lung Perfusion Using a Split Human Donor Model
- Liproxstatin-1 Alleviates Lung Transplantation-induced Cold Ischemia–Reperfusion Injury by Inhibiting Ferroptosis
Original Clinical Science—Liver
- Preventing Small-for-size Syndrome in Living Donor Liver Transplantation: Guidelines From the ILTS-iLDLT-LTSI Consensus Conference
- Anesthesia and Critical Care for the Prediction and Prevention for Small-for-size Syndrome: Guidelines from the ILTS-iLDLT-LTSI Consensus Conference
- Post Living Donor Liver Transplantation Small-for-size Syndrome: Definitions, Timelines, Biochemical, and Clinical Factors for Diagnosis: Guidelines From the ILTS-iLDLT-LTSI Consensus Conference
- Management of Established Small-for-size Syndrome in Post Living Donor Liver Transplantation: Medical, Radiological, and Surgical Interventions: Guidelines From the ILTS-iLDLT-LTSI Consensus Conference
- The Accuracy of Nonstandardized MELD/PELD Score Exceptions in the Pediatric Liver Allocation System
Original Clinical Science—General
- Evaluation of Immunocompetence and Biomarkers of Tolerance in Chimeric and Immunosuppression-free Kidney Allograft Recipients
- Immunized Patients Face Reduced Access to Transplantation in the Eurotransplant Kidney Allocation System
- The Association Between Frailty and Chronic Lung Allograft Dysfunction After Lung Transplantation
- Relative Lung Perfusion on Ventilation–Perfusion Scans After Double Lung Transplant
- Performance and Advancement of the Kidney Solid Organ Response Test
- Seroprevalence of Measles, Mumps, Rubella, and Varicella-Zoster Virus and Seroresponse to the Vaccinations in Adult Solid Organ Transplant Candidates
Letters to the Editor
- The Problem and the Solution for Equitable Access of HLA-DR Homozygous Patients to Kidney Transplantation
- New Desensitization Strategy: Daratumumab for Highly Sensitized Pediatric Heart Transplant Candidate
- The Virtual Crossmatch: What’s in a Name?
- Virtual Crossmatch: By Any Other Name
- The Transplantgram Revolution: Instagram’s Influence on the Perception and Promotion of Organ Transplantation
- Clinical Outcomes Among Kidney Transplant Recipients During Omicron XBB Contrasted Against Preceding BA.1, BA.2, and BA.4/5 Pandemic Waves
- Humoral Response After 6 or More Successive Doses of SARS-CoV-2 mRNA Vaccines in Kidney Transplant Recipients—Should We Keep Vaccinating?
News Spotlight
TTS-ISN Sister Centers Application Submission - DEADLINE APPROACHING!
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!