2021 TTS Needs Assessment Survey
Be Part of TTS’ Future!
We are asking our members to help us understand how TTS can better serve you!
Take the TTS 2021 Needs Assessment Survey.
Results of the survey will directly impact TTS activities!
Participate by: February 12, 2021
Click here to take the survey «HOT OFF THE PRESS»
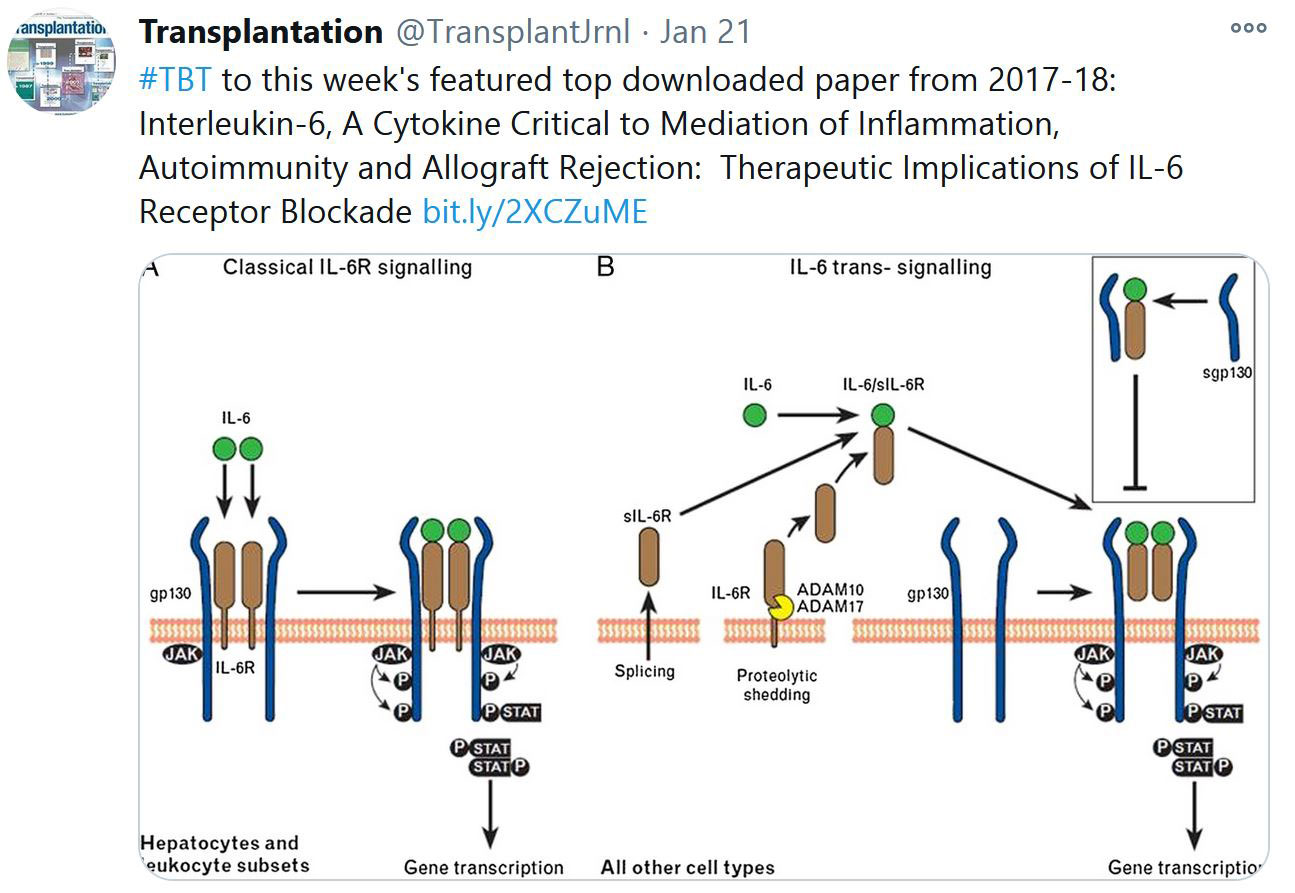
RECENT PUBLICATIONS IDENTIFIED BY TTS EDUCATION COMMITTEE ON COVID-19
Selected Publications by TTS Education Committee. This week's selection made by Enver Akalin, Marlies Reinders and Millie Samaniego.
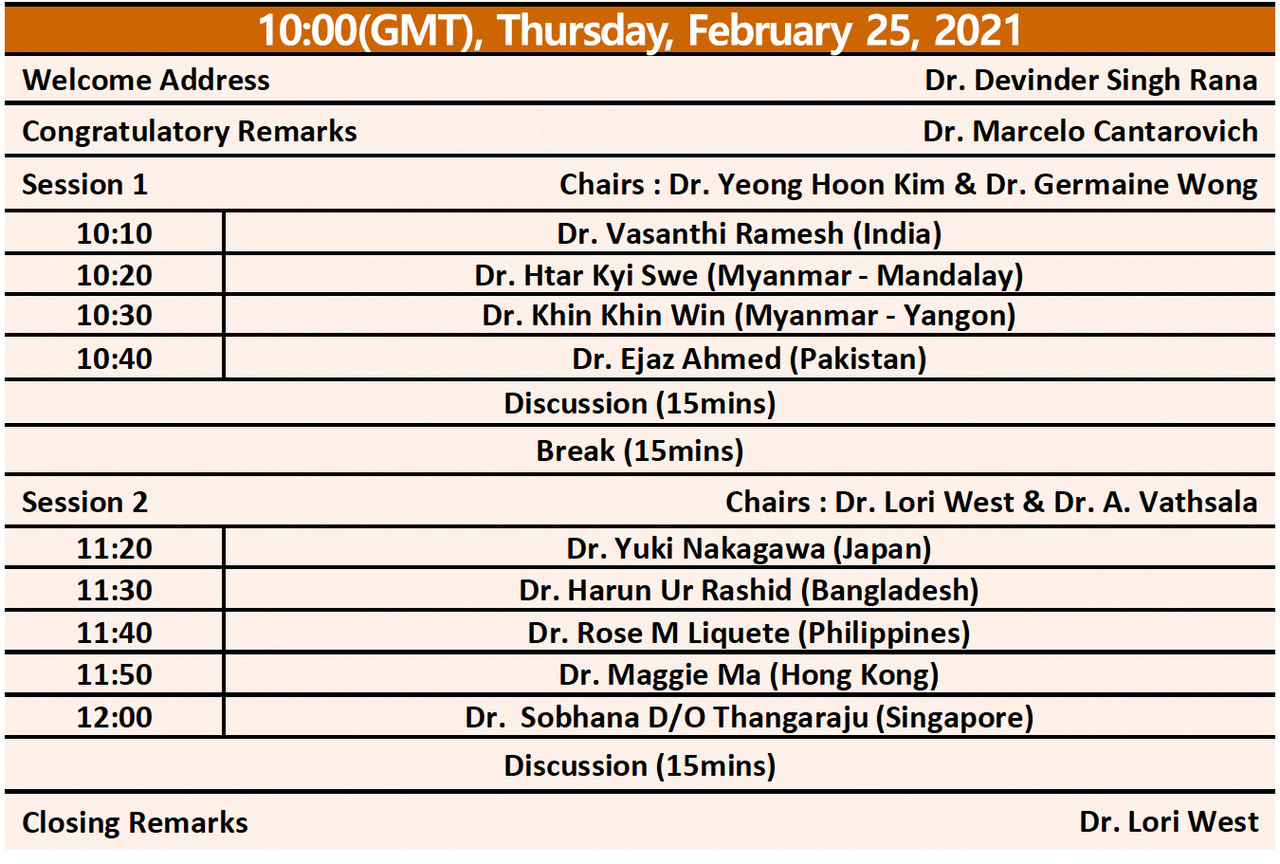
TTS-ILTS Paired Transplant Centers Program
Application Deadline Extended to February 24th, 2021
The TTS-ILTS Paired Transplant Centers Program is a collaboration between The Transplantation Society (TTS) and the International Liver Transplantation Society (ILTS) supporting new liver transplant programs in emerging economies.

TTS and TTS Sections News
Members were emailed their invoices a month ago.
If you are not a member click the Join TTS button below!
CORONAVIRUS (COVID-19) UPDATES
IN THE NEWS
UPCOMING MEETINGS AND ANNOUNCEMENTS
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!