New! Joint IXA-CTRMS 2021 Virtual Congress

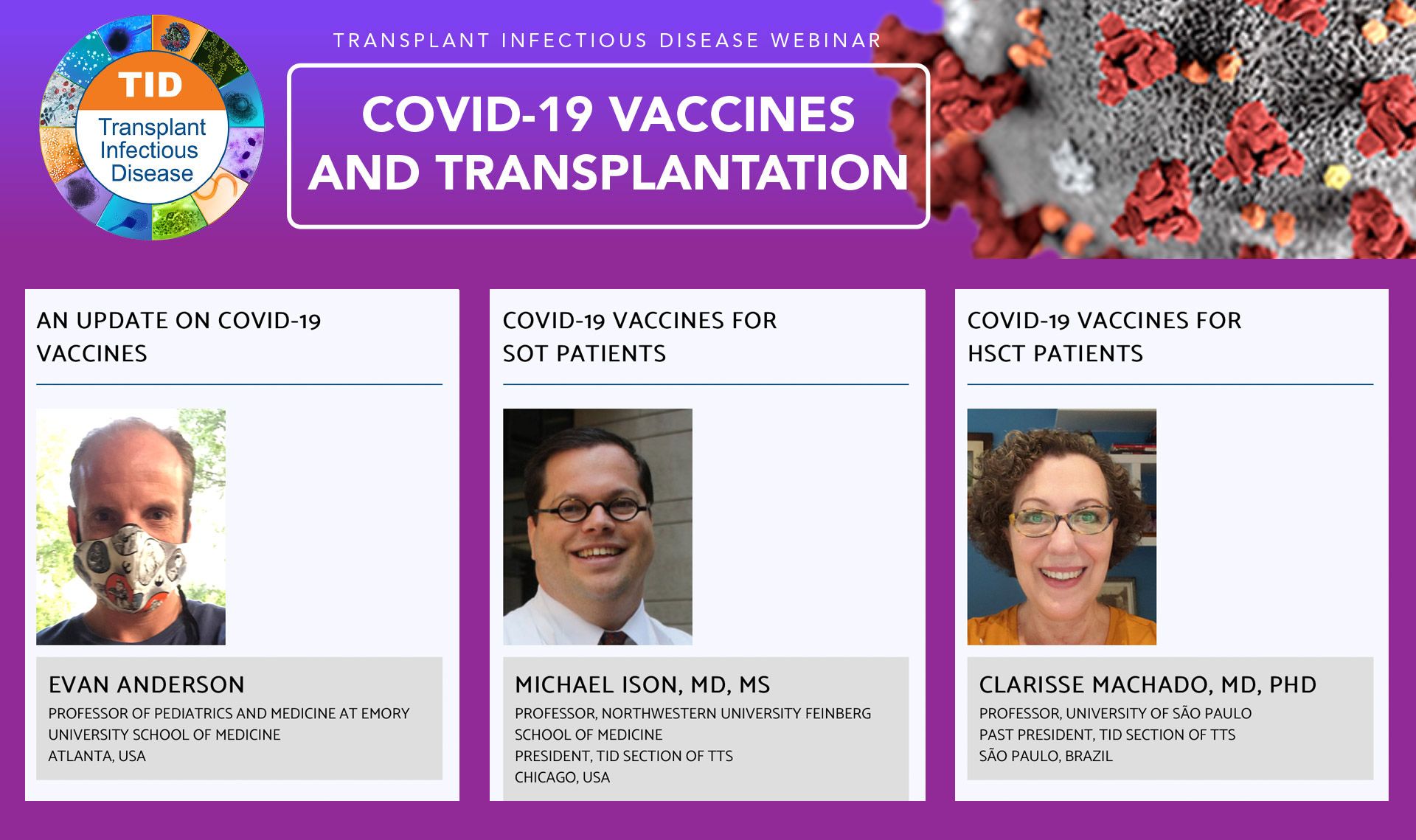
TRANSPLANT INFECTIOUS DISEASE WEBINAR
COVID-19 VACCINES AND TRANSPLANTATION
Open to all healthcare professionals
The emergence of the SARS-CoV-2 pandemic has resulted in substantial morbidity and mortality in the US and worldwide. Vaccines have been developed in record time, with several receiving FDA EUA approval. Understanding the role, features, and side effects of these various vaccines is important to help inform use and expectations. Data are lacking in the transplant population, but physicians should be aware of key principles for protecting their patients.

Notice of Postponement
ISN-TTS JOINT WEBINAR
Donor/Recipient Pair:
RISKS VS. GAINS - KIDNEY FUNCTION
Open to all healthcare professionals
Speaker: Marcelo Cantarovich, TTS President
Moderator: Jean Tchervenkov, Montreal, Canada
Due to unforseen circumstances thsi webinar is postponed
A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!