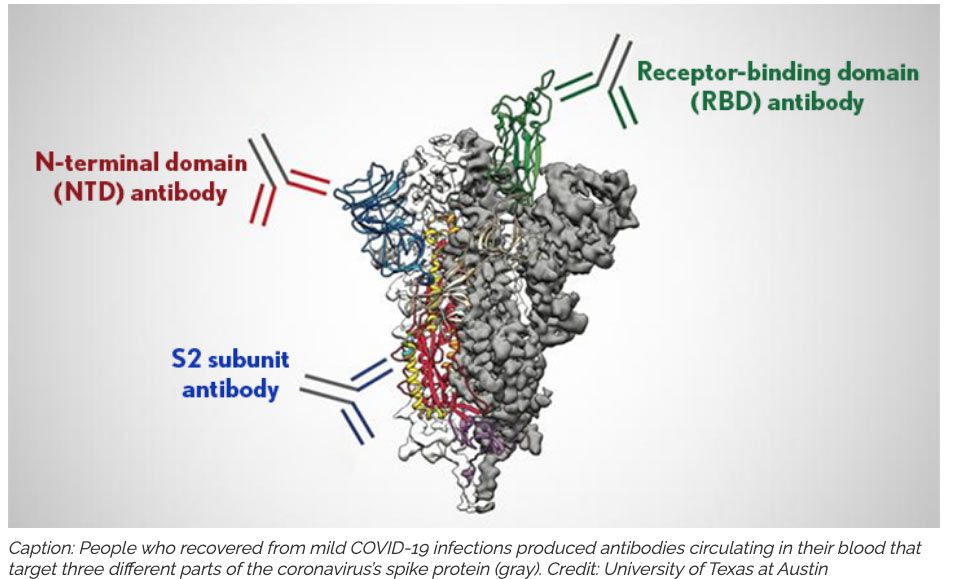
Statement on COVID-19 Vaccination in Solid Organ Transplant Recipients

Join us for the TTS Masterclass Series
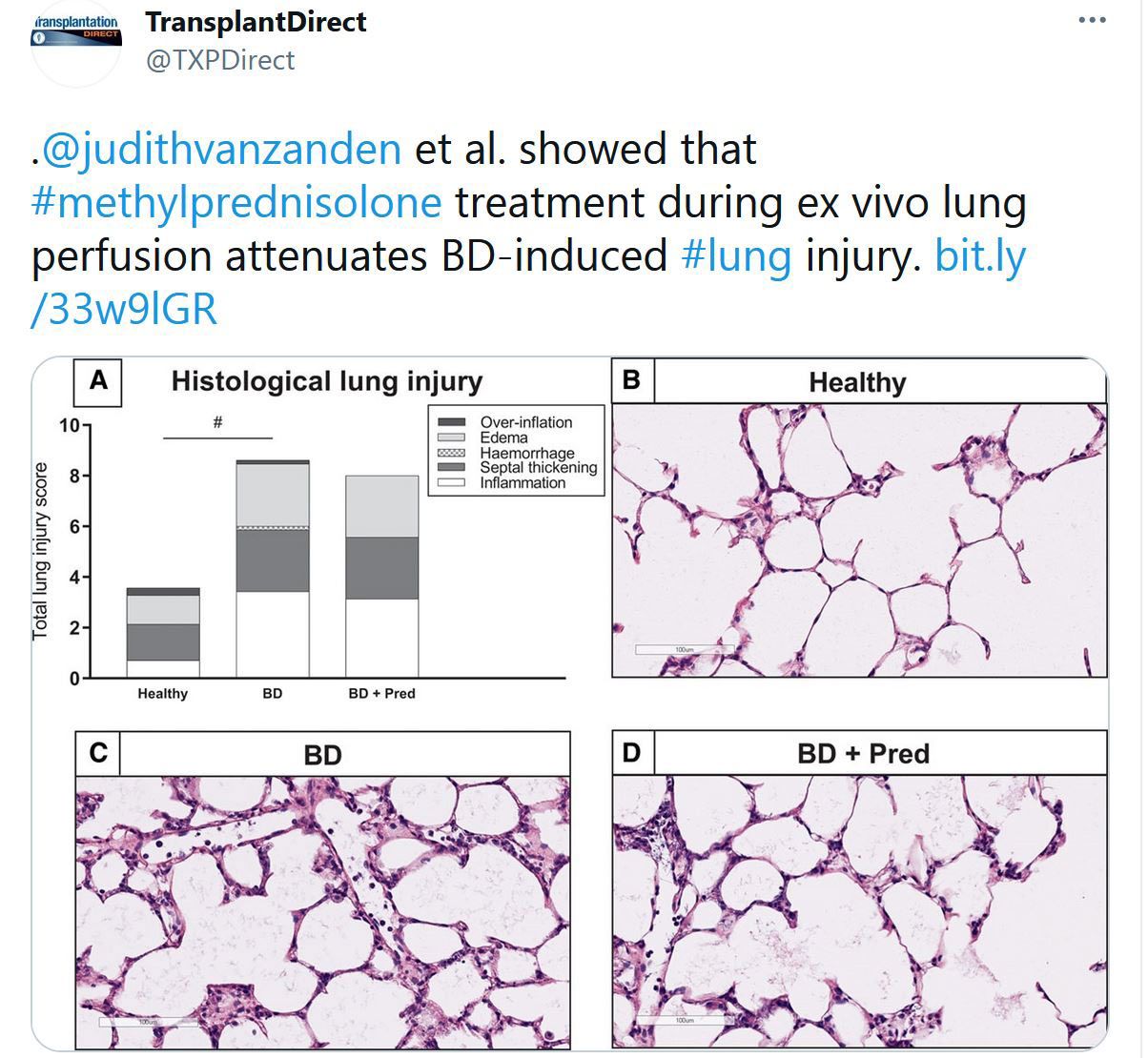
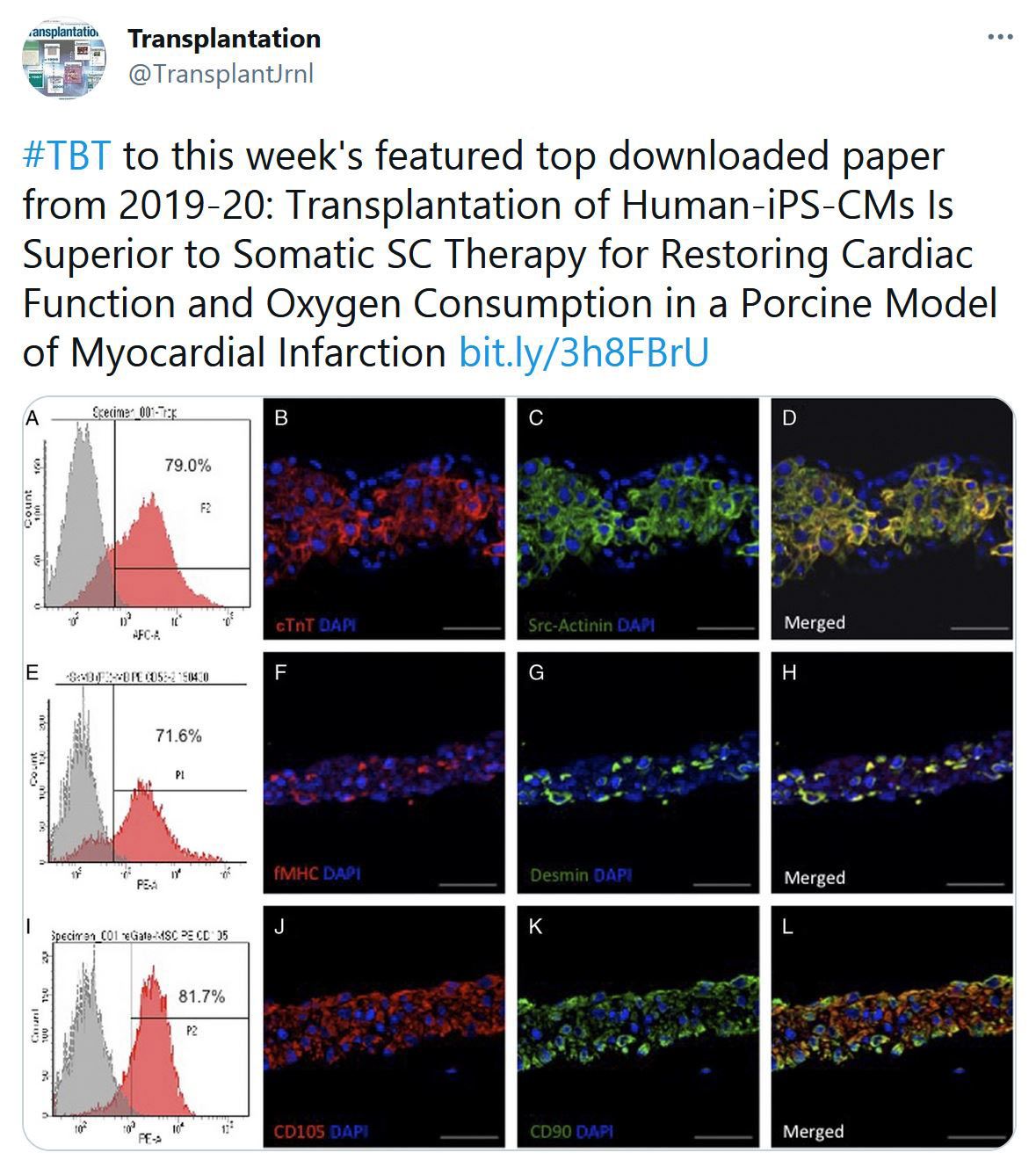
Highlighted Tweets
Webinars (Upcoming and Recordings)

IXA-CTRMS are pleased to announce the extension of the abstract submission deadline until June 1, 2021.
We thank all authors from across the world who have already completed their submission. Please note that there will be no further extension to the deadline.
For guidelines and to submit your abstract click here
New Course from the DICG

Highlighted News
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!