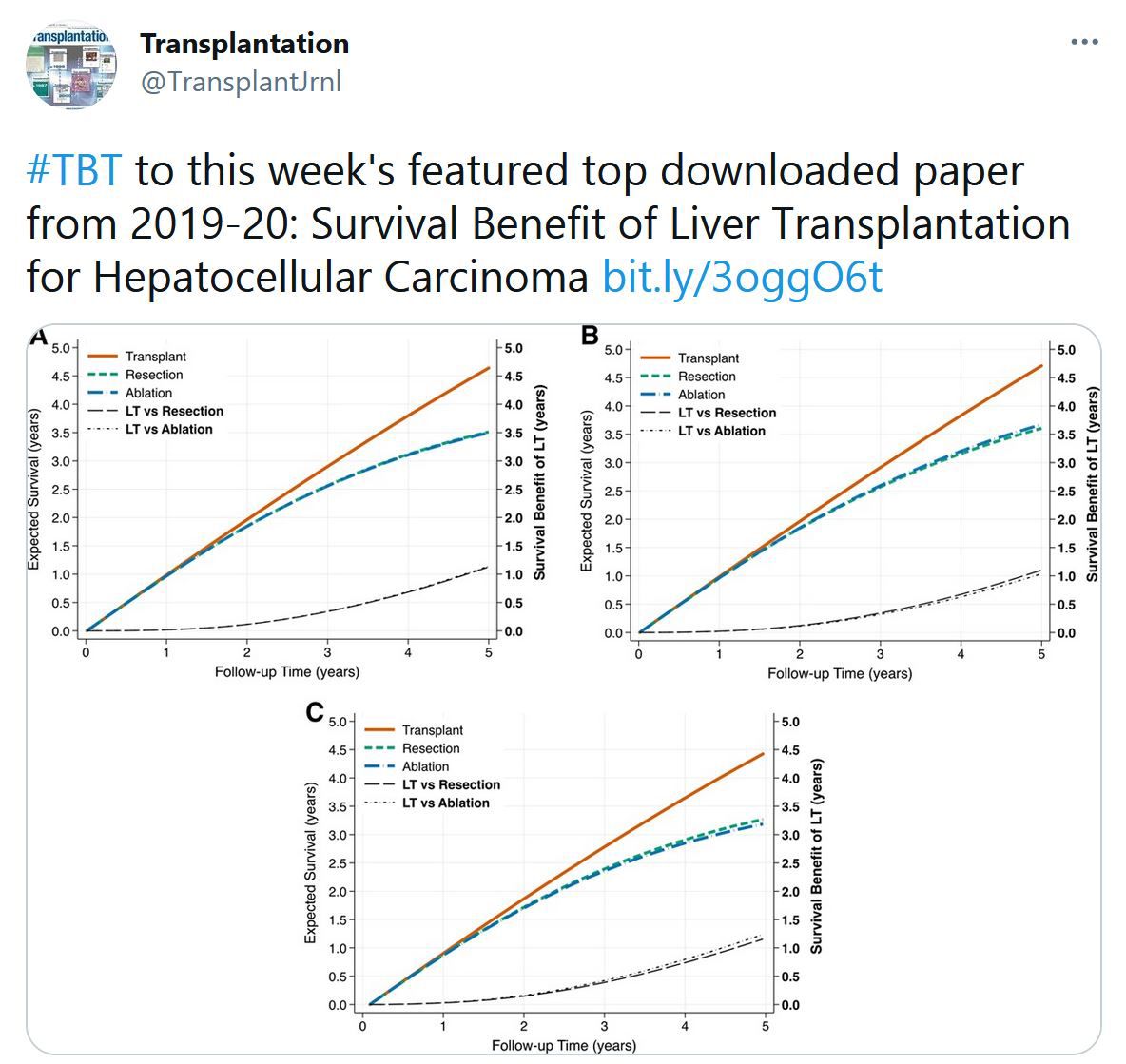
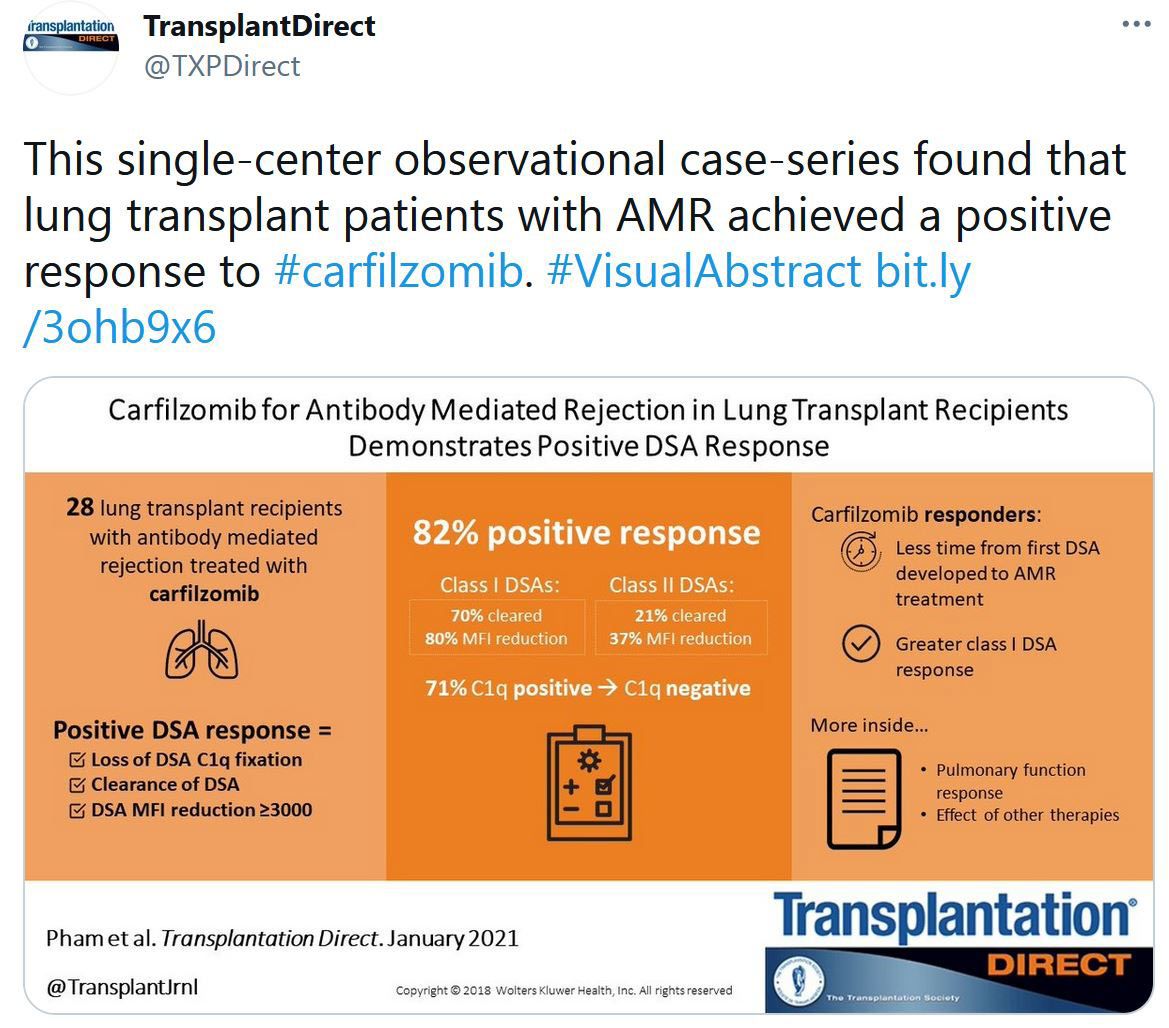
Statement on COVID-19 Vaccination in Solid Organ Transplant Recipients
New Endorsing Organizations Added!
Recently, multiple studies have been published examining the response to SARS-CoV-2 mRNA-based vaccines in solid organ transplant (SOT) recipients.(1-7) Overall, these have demonstrated reduced antibody responses to vaccine when compared with reports involving the general public.
The low antibody response rate is concerning but not unexpected as SOT recipients have lower rates of immune responses to other vaccines as well.(8) Further data are needed to evaluate B- and T- cellular responses in SOT recipients after SARS-CoV-2 vaccination and to assess vaccine effectiveness particularly for protection against severe COVID-19 as a clinical end-point. Previous experience with influenza vaccination in transplant patients has demonstrated reduced influenza-related lower respiratory tract disease and hospitalization despite low antibody response.(9, 10). While breakthrough cases of COVID-19 after partial or full vaccination in SOT recipients may occur, it is important to recognize that we may be preventing more cases or reducing severity through vaccination.(11, 12) Thus, we strongly caution against concluding that low antibody response rate to SARS-CoV-2 vaccination will lead to reduced clinical effectiveness until more information is available. These results should not prompt or encourage vaccine hesitancy in SOT recipients.
Immunosuppressed patients are known to have prolonged viral shedding of actively replicating virus which may promote the development of viral variants.(13) Additionally, there are data to suggest worse outcomes in SOT recipients with COVID-19 compared to the general population.(14) The effect of immunization on duration of viral shedding and clinical outcomes remains unknown for this population.
Until more complete data are available, we urge:
- Pre-transplant vaccination of all SOT candidates as a priority whenever feasible.
- Continued SARS-CoV-2 vaccination in SOT recipients and priority for vaccination of their household members and caregivers to reduce exposure risk for these vulnerable patients.
- Continuation of a stable immunosuppression regimen at the time of vaccination to avoid the risk of organ rejection until more comprehensive data are available.
- Continued adherence of all transplant recipients to protective measures including masking and social distancing regardless of vaccination status.
- Boyarsky BJ, Werbel WA, Avery RA, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM: Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021.
- Benotmane I, Gautier-Vargas G, Cognard N, et al.: Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int 2021.
- Sattler A SE, Weber U, Potekhin A, Bachmann F, Budde K, Storz E, Proß V, Bergmann Y, Thole L, Tizian C, Hölsken O, Diefenbach A, Schrezenmeier H, Jahrsdörfer B, Zemojtel T, Jechow K, Conrad C, Lukassen S, Stauch D, Lachmann N, Choi M, Halleck F, Kotsch K. MedRxv. doi: https://doi.org/10.1101/2021.04.06.21254963. Accessed 4/19/2021: Impaired Humoral and Cellular Immunity after SARS-CoV2 BNT162b2 (Tozinameran) Prime-Boost Vaccination in Kidney Transplant Recipients.
- Yi SG, Knight RJ, Graviss EA, et al.: Kidney Transplant Recipients Rarely Show an Early Antibody Response Following the First COVID-19 Vaccine Administration. Transplantation 2021.
- Peled Y RE, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, Lustig Y, Rahav G: BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant 2021.
- Narasimhan M ML, Clark AE, Usmani A, Cao J, Raj E, Torres F, Sarode R, Kaza V, Lacelle C, Muthukumar A: Serological Response in Lung Transplant Recipients after Two Doses of SARS-CoV-2 mRNA Vaccines. medRxiv 2021.
- Shostak Y SN, Heching M, Rosengarten D, Shtraichman O, Shitenberg D, Amor SM, Yahav D, Zvi HB, Pertzov B, Kramer MR: Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. The Lancet Resp Med 2021.
- Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T: Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One 2013;8:e56974.
- Kumar D, Ferreira VH, Blumberg E, et al.: A 5-Year Prospective Multicenter Evaluation of Influenza Infection in Transplant Recipients. Clin Infect Dis 2018;67:1322-9.
- Pinana JL, Perez A, Montoro J, et al.: Clinical Effectiveness of Influenza Vaccination After Allogeneic Hematopoietic Stem Cell Transplantation: A Cross-sectional, Prospective, Observational Study. Clin Infect Dis 2019;68:1894-903.
- Basic-Jukic N, Jelicic I: SARS-CoV-2 infection after two doses of mRNA vaccine in renal transplant recipients. Transpl Infect Dis 2021:e13628.
- Wadei HM GT, Leoni JC, Shah SZ, Aslam N, Speicher LL., COVID-19 infection in Solid Organ Transplant Recipients after SARS-CoV-2 vaccination. Am J Transplant 2021.
- Aydillo T, Gonzalez-Reiche AS, Aslam S, et al.: Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med 2020;383:2586-8.
- Nair V, Jandovitz N, Hirsch JS, et al.: An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients. Am J Transplant 2020.
Memorial Service for Agnes M. Isabey-Azimzadeh

Dear Colleagues,
Agnès Azimzadeh passed away on March 15, 2021, after a courageous battle with intestinal cancer. The scientific and clinical transplant communities she touched at Massachusetts General Hospital, University of Maryland, and around the world join her family in celebrating her life, and mourning her premature loss.
On Saturday, May 29th at 10:00AM EDT, her professional colleagues and former trainees will gather virtually to celebrate Agnes's life and her scientific contributions. A link to the gathering can be found at:
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!