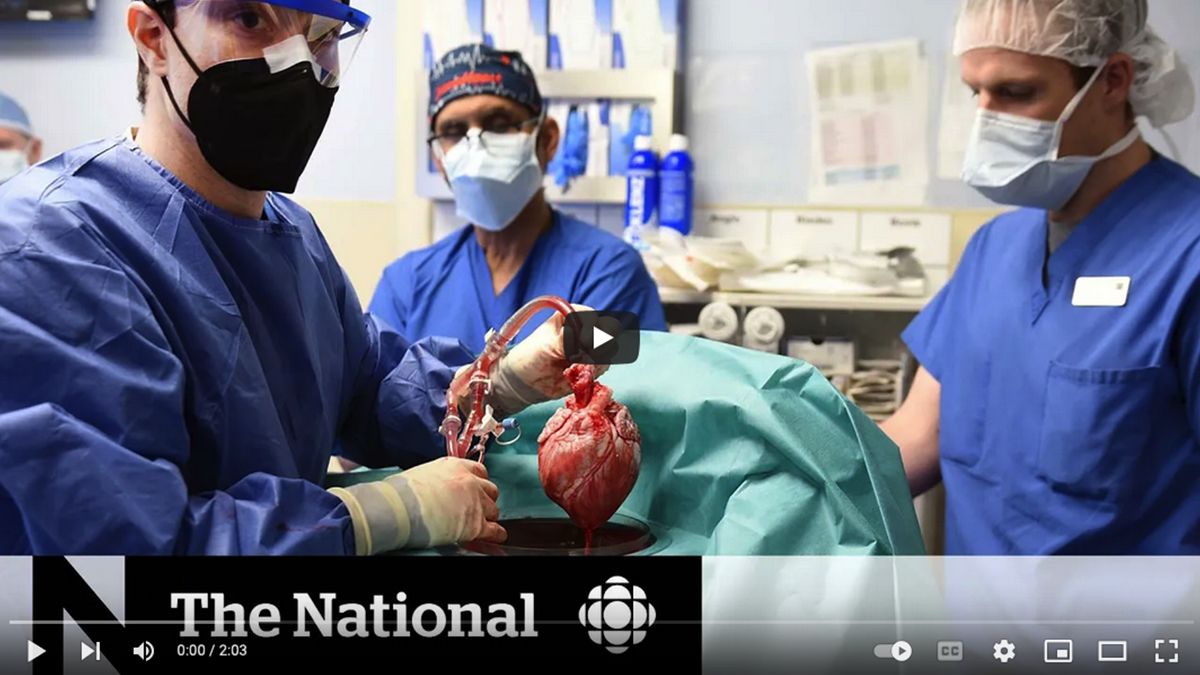
Man Receives a Heart From a Genetically Altered Pig
Articles from around the world regarding this achievement:

2022 TTS Call for Nominations for Officers and Councilors
In 2022, three Officer positions will be vacated and 5 of the 12 Councilors-at-large representing the Regions will be changing. The elections will take place early in 2022 and those elected will assume their new roles starting at the 2022 TTS Congress in Buenos Aires.

Members can access the online nominating form at www.tts.org/nominations. Since each nominee must have his or her form signed by three supporting members (including him/herself), the online process allows for efficient and rapid circulation.
THE NOMINATION DEADLINE IS FEBRUARY 14, 2022.
Please note:
- As successive presidents may not be from the same Region, members from the Middle East / Africa region who would have otherwise been eligible to become President-Elect are not eligible in these elections.
- Only members who have served a full term on Council and have paid their dues are eligible for the Officer positions (President-Elect, Secretary and Treasurer).
- Only full members who have paid these dues are eligible to be nominated for Council.
- Only full members who have paid their dues may nominate and/or vote.
For more information on elections, visit the TTS website and consult the By-Laws in the “About” section.
Nominations are being sought for these positions
Officer Positions:
- President-Elect
- Vice President
- Treasurer
TTS Regions:
- Europe (1)
- Latin America (1)
- Middle East / Africa (1)
- North America (1)
- Oceania (1)
2022 Awards Call for Nominations

Call for nominations
Recognized as the world's highest dedicated award for the most outstanding contributions in the field of transplantation.
The Medawar Prize
The Medawar Prize, named after Society co-founder Sir Peter Medawar, is recognized as the world's highest dedicated award for the most outstanding contributions in the field of transplantation. The Medawar Prize has been awarded at each of our Society's biennial Congresses since 1990.
The award recognizes the outstanding investigators whose contributions have had such a profound influence on the field of organ transplantation. The Medawar Prize is universally considered to be commensurate with the most outstanding world prizes for scientific achievement.
May 1, 2022
Eligibility to Award
Frequency of Award
Selection of the Recipient
Monetary Award
Past Medawar Laureates

CALL FOR NOMINATIONS
Previous Winners

Call for nominations
Available Awards for 2022
Outstanding Achievement Transplantation Science (Basic)
Outstanding Achievement in Transplantation Science (Clinical)
Outstanding Achievement in Transplantation Science (Developing Country)
Mentorship or Education and Training in Transplantation

THE INTERNATIONAL TRANSPLANTATION SCIENCE MENTEE-MENTOR AWARDS FOR THE 29TH INTERNATIONAL CONGRESS OF THE TRANSPLANTATION SOCIETY (TTS 2022) – BUENOS AIRES, ARGENTINA, SEPTEMBER 10-14, 2022.
The Transplantation Society celebrates the contributions of basic science to the field of transplantation with the International Transplantation Science Mentee-Mentor Awards to recognize the efforts of scientists who have advanced our understanding of transplantation science and fostered the development of the young investigators who will be the future leaders in our field.
The application will open in January 2022. Deadline for submission is April 10, 2022.
Click here for more detailsTTS Early Career Members Committee - Quick Clip Interview
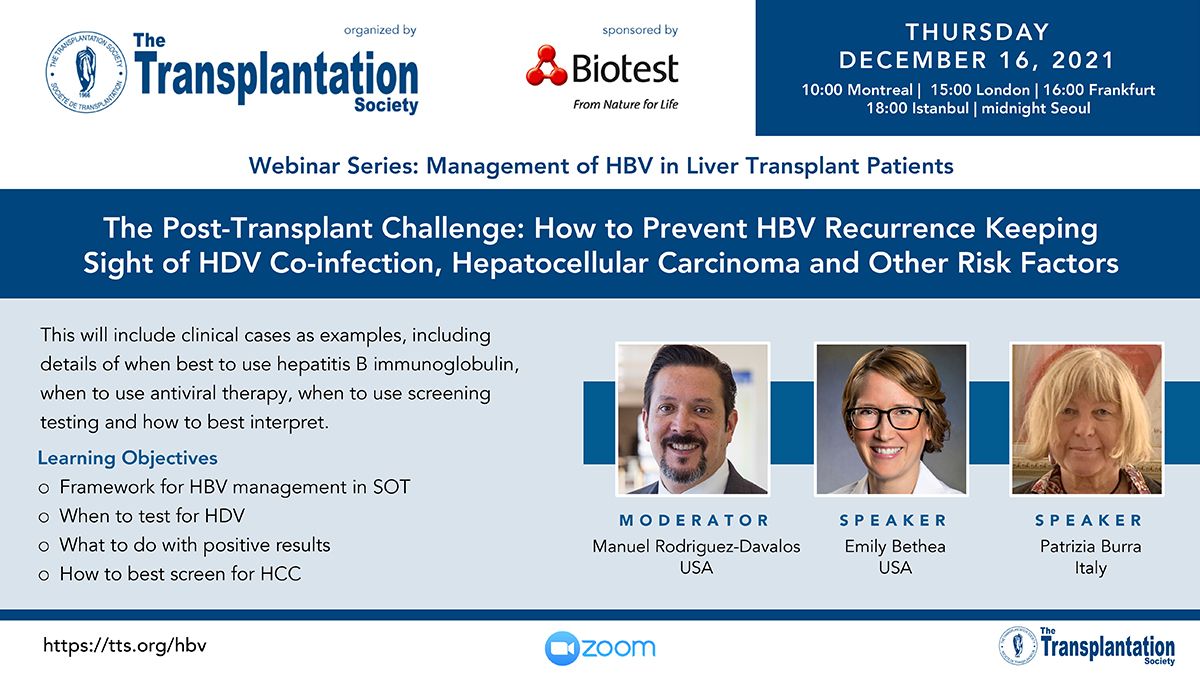
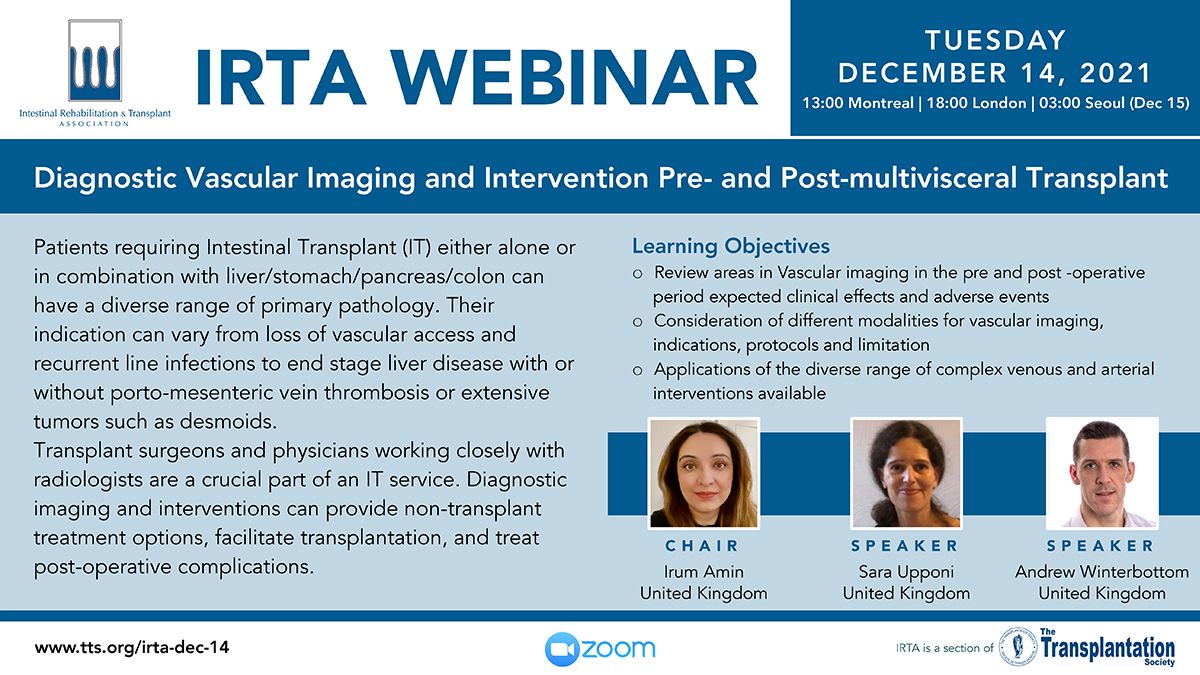
Upcoming Webinar Presentations

- Antibody responses to COVID-19 vaccines in transplant recipients are diminished compared with the general population.1-16 However,
- The level of protective antibody has yet to be defined. Based on data derived from trials in the general population, there is an inverse correlation between the level of neutralizing antibody to SARS-CoV-2 spike protein and symptomatic disease. Additionally, the ability of some monoclonal antibodies against spike proteins to attenuate disease shows the protective nature of antibody. While this is true for populations, the individual antibody response remains unpredictable.
- Three dose mRNA vaccination is associated with improved rates of serum neutralization against SARS-CoV-2 variants in transplant recipients versus 2-dose vaccination.17
- The post-vaccination antibody threshold for protection against severe COVID-19 is significantly lower than that required to prevent viral infection.18
- Determination of protective levels of antibody is confounded by the wide variety of antibody tests that are commercially available, with no direct means to compare results from the different tests.
- The protective components of Cellular (T cell and NK T cells) and humoral responses (IgG/IgM vs IgA) may not be linked in individual SOT recipients; it is possible to have an active acquired or innate immune response in the absence of antibody and vice versa.3,6,9-11,16 However, the clinical consequence of this divergence is not known nor measurable.
- Antibody testing is currently not recommended by the FDA due to the following considerations:
- Most commercially available tests do not examine neutralizing antibody to the spike protein receptor binding domain (RBD).
- Many commercially available tests are qualitative.
- The analytical cut-off values for antibody detection are not necessarily the same as clinically relevant values.
- There is no commonly agreed upon titer that has been defined as protective against SARS-CoV-2 infection.
- Cellular responses may occur in the absence of measurable antibody
- However, failure to mount any detectable antibody response to the spike protein after vaccination is concerning for increased risk for COVID-19 acquisition, especially with more transmissible variants.
- Clinical effectiveness studies in SOT show an overall reduction in infections after 2-dose vaccination and an effectiveness of 59% (in a CDC study that combined the SOT and stem cell transplant population).19,20 v. 8 11.29.2021.
Moreover, breakthrough infections occur at greater rates and greater severity than in the general population.21 - While the level of immunosuppression, specifically the use of antiproliferative agents, has been implicated as a factor in poor antibody response after vaccination, there is no reliable guide for adjustment of immunosuppression in anticipation of vaccine responses. Current data suggest that providing a third dose of mRNA vaccine to SOT recipients that have previously received two doses of mRNA vaccine can increase antibody titers to SARS-CoV-2;22-25 in a recent, double-blind, randomized placebo-controlled trial, a third dose mRNA vaccine provided at an interval of two months after the second dose significantly increased antibody titers, neutralizing antibody, and cellular immune response to SARS-CoV-2 compared to third dose placebo.26 For individuals who have received a single dose of an adenovirus vector vaccine, a second vaccine dose with either the same vaccine or an mRNA vaccine is indicated for all individuals, including transplant candidates and recipients. It is unknown if a specific vaccine is preferable for this additional dose. Early data from the UK in healthy individuals suggests that using an mRNA vaccine may be preferred to addition adenoviral-vector vaccine doses though transplant-specific data is not available.27/li>
- The published data to date suggest that additional doses are safe and reasonably well-tolerated with no evidence of an increased risk of rejection attributable to vaccine.
- Clinical effectiveness of expanded vaccine dosing is pending. There are insufficient data to recommend the use of antibody testing to guide decision-making about additional doses. The effect of additional vaccine doses for vector-based and other vaccines is not clear.
- Many of the reports to date focus on kidney transplant recipients, but it does appear that other organ recipients experience similar responses to additional vaccine doses. Despite 3 doses of mRNA vaccine, there are still patients that have poor antibody responses and we do not know what interventions, if any, might be indicated. Whether altering the vaccine used for the additional dose (e.g. giving adenovirus vector following mRNA or vice versa) is unknown.
- We strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine that is approved or authorized in their jurisdiction.
- Whenever possible, vaccination should occur prior to transplantation (ideally with completion of vaccine series a minimum of 2 weeks prior to transplant).
- We support the development of institutional policies regarding pre-transplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and post-transplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.
- SOT recipients that have received 2-dose mRNA vaccine should also receive a third dose of mRNA vaccine to complete the primary series.
- Further research is needed to clarify if additional doses of other vaccines, including adenoviral vector vaccines will provide similar benefit. At this time, patients who have received a single dose of adenovirus vector vaccine (typically Johnson &Johnson/Janssen) would benefit from a second dose of vaccine and data from immunocompetent hosts suggest that that an mRNA vaccine dose may offer v. 8 11.29.2021 benefits. Consequently, we recommend that mRNA vaccine be considered for transplant recipients that previously received an adenoviral vector vaccine. There are limited data to provide guidance regarding additional doses of COVID-19 vaccine for those who have received 2 doses of Astra Zeneca based adenovirus vaccine, however it is reasonable to provide 3rd doses for these individuals and mRNA vaccines can be used in this circumstance.
- There are no data currently to support adjustment of immunosuppression in anticipation of additional doses of vaccination.
- Antibody testing is not required prior to or after additional vaccine doses and is not currently recommended by the FDA.
- However, individual physicians and patients may decide that antibody testing is desirable to discern the individual patient’s response to the vaccine antigen with a resultant discussion about the interpretation of the test results and the consequences/risks of acquiring COVID-19 infection. The discussion will need to include additional issues relevant to the patient, such as local prevalence of SARS-CoV-2 and its variants, personal situations relating to immunosuppression and transplant infections and the vaccination level in the household that may be pertinent.
- It is strongly recommended that living donors be vaccinated with a minimum of two-doses of vaccine and boosters are encouraged. This is to minimize peri-operative risks for donor (and spread to the recipient if there is contact). 28
- All eligible household and close contacts of SOT recipients should be vaccinated against SARS-CoV-2 to minimize risks to the recipient.
- It is strongly recommended that all health care providers be vaccinated against SARS-CoV-2 to foster a safer environment for our patients and maintain a skilled workforce.
- While COVID-19 variants continue to evolve and circulate in the community and the extent of protection is still unknown in transplant recipients, it is recommended that SOT candidates and recipients continue to adhere to protective measures including masking in public spaces, social distancing, frequent hand washing, and avoiding indoor crowds. These measures should be followed regardless of whether the patient has received additional doses of vaccine. Information about viral variants, vaccine effectiveness and perceived/real risk is changing rapidly and it is important to address the concerns of transplant recipients.
- We recommend ongoing monitoring of the regulatory and health department websites to obtain up to date COVID-19 prevalence and vaccine updates.
- We strongly encourage participation in clinical studies to determine the effects of additional doses or other strategies to improve vaccine responses.
- We strongly urge funding agencies to invest in research evaluating vaccine immunogenicity, vaccine effectiveness, and strategies to enhance vaccine responses in vulnerable populations, including SOT candidates and recipients, who may fuel the perpetuation of the pandemic.
- Brian J. Boyarsky WAW, Robin K. Avery, Aaron A. R. Tobian, Allan B. Massie, Dorry L. Segev, Jacqueline M. Garonzik-Wang. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021.
- Sattler A SE, Weber U, Potekhin A, Bachmann F, Budde K, Storz E, Proß V, Bergmann Y, Thole L, Tizian C, Hölsken O, Diefenbach A, Schrezenmeier H, Jahrsdörfer B, Zemojtel T, Jechow K, Conrad C, Lukassen S, Stauch D, Lachmann N, Choi M, Halleck F, Kotsch K. MedRxv. doi: https://doi.org/10.1101/2021.04.06.21254963. Accessed 4/19/2021. Impaired Humoral and Cellular Immunity after SARS-CoV2 BNT162b2 (Tozinameran) Prime-Boost Vaccination in Kidney Transplant Recipients. Accessed.
- Yi SG, Knight RJ, Graviss EA, et al. Kidney Transplant Recipients Rarely Show an Early Antibody Response Following the First COVID-19 Vaccine Administration. Transplantation. 2021.
- Peled Y RE, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, Lustig Y, Rahav G. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibodyresponse. J Heart Lung Transplant. 2021.
- Havlin J SM, Dvorackova E et al. . Immunogenicity of BNT162b2 mRNA COVID19 Vaccine and SARS-CoV-2 Infection in Lung Transplant Recipients. Journal of Heart and Lung Transplantation. 2021.
- Narasimhan M ML, Clark AE, Usmani A, Cao J, Raj E, Torres F, Sarode R, Kaza V, Lacelle C, Muthukumar A. Serological Response in Lung Transplant Recipients after Two Doses of SARS-CoV-2 mRNA Vaccines. medRxiv. 2021.
- Shostak Y SN, Heching M, Rosengarten D, Shtraichman O, Shitenberg D, Amor SM, Yahav D, Zvi HB, Pertzov B, Kramer MR. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. The Lancet Resp Med. 2021.
- Miele M, Busa R, Russelli G, et al. Impaired anti-SARS-CoV-2 Humoral and Cellular Immune Response induced by Pfizer-BioNTech BNT162b2 mRNA Vaccine in Solid Organ Transplanted Patients. Am J Transplant. 2021.
- Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021.
- Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021.
- Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(12):3980-3989.
- Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021.
- Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-Cov-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021.
- Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients - a prospective cohort study. Eur J Heart Fail. 2021.
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211.
- Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021. v. 8 11.29.2021
- Kumar D, Ferreira VH, Hall VG, et al. Neutralization of SARS-CoV-2 Variants in Transplant Recipients After Two and Three Doses of mRNA-1273 Vaccine : Secondary Analysis of a Randomized Trial. Ann Intern Med. 2021.
- Basic-Jukic N, Jelicic I. SARS-CoV-2 infection after two doses of mRNA vaccine in renal transplant recipients. Transpl Infect Dis. 2021:e13628.
- Aslam S, Adler E, Mekeel K, Little SJ. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021:e13705.
- Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults - Nine States, January-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1553-1559.
- Qin CX, Moore LW, Anjan S, et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation. 2021;105(11):e265-e266.
- https://www.nih.gov/news-events/news-releases/nih-clinical-trial-evaluating-mixed-covid-19-vaccine-schedules-begins [press release]. 6/1/2021.
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021.
- Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann Intern Med. 2021.
- Stumpf J, Tonnus W, Paliege A, et al. Cellular and Humoral Immune Responses After 3 Doses of BNT162b2 mRNA SARS-CoV-2 Vaccine in Kidney Transplant. Transplantation. 2021;105(11):e267-e269.
- Hall VG, Ferreira VH, Ku T, et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021.
- Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856-869.
- Lancet: May 29, 2020 DOI: https://doi.org/10.1016/S0140-6736(20)31182-X
Survey of Transplant Center Practices related to COVID-19 Vaccine Mandates
Women in Transplantation Fellowship Grant
Letters of Intent are due by February 1, 2022
With the support of Industry Partners, WIT will provide funding to support research to early career researchers that contributes to the the Grant Recipient's understanding of the impact of sex and gender issues in solid organ transplantation and immunology. The spectrum of studies includes epidemiological, basic, clinical and translational.
Please go to www.tts-wit.org/2022_fellowship_grant or email Katie Tait, TTS Special Initiative Manager at katie.tait@tts.org for more information.
Latest Video Additions
TTS-ILTS Paired Transplant Centers Program
Extended Deadline: February 1, 2022
The TTS-ILTS Paired Transplant Centers Program is now accepting applications!
The TTS-ILTS Paired Transplant Centers Program is a collaboration between The Transplantation Society (TTS) and the International Liver Transplantation Society (ILTS) supporting new liver transplant programs in emerging economies.
Symposium Program Now Available!
The program is now available as a PDF Document!
Contact
Address
The Transplantation Society
International Headquarters
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada
Используйте Вавада казино для игры с бонусом — активируйте промокод и начните выигрывать уже сегодня!