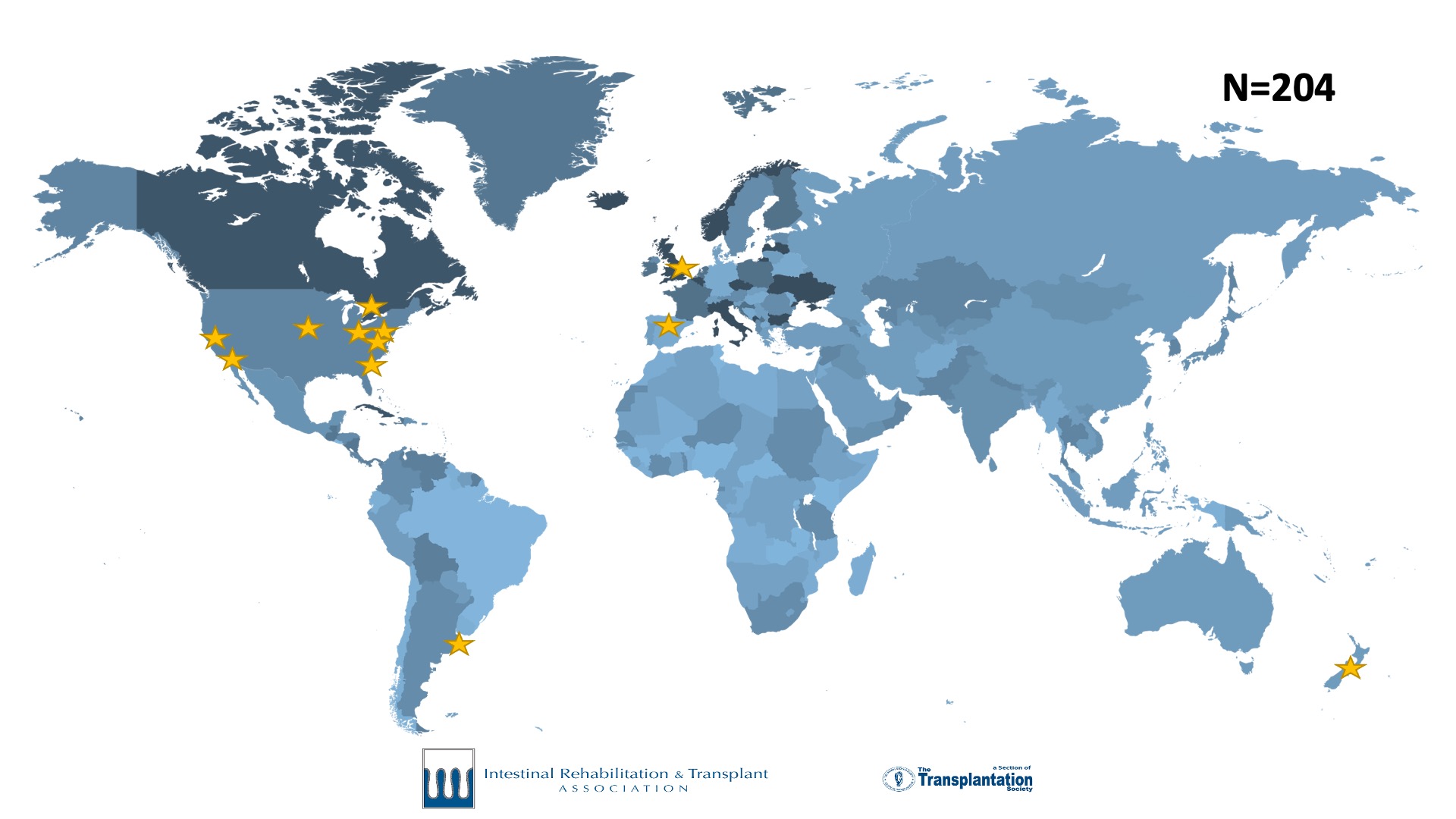
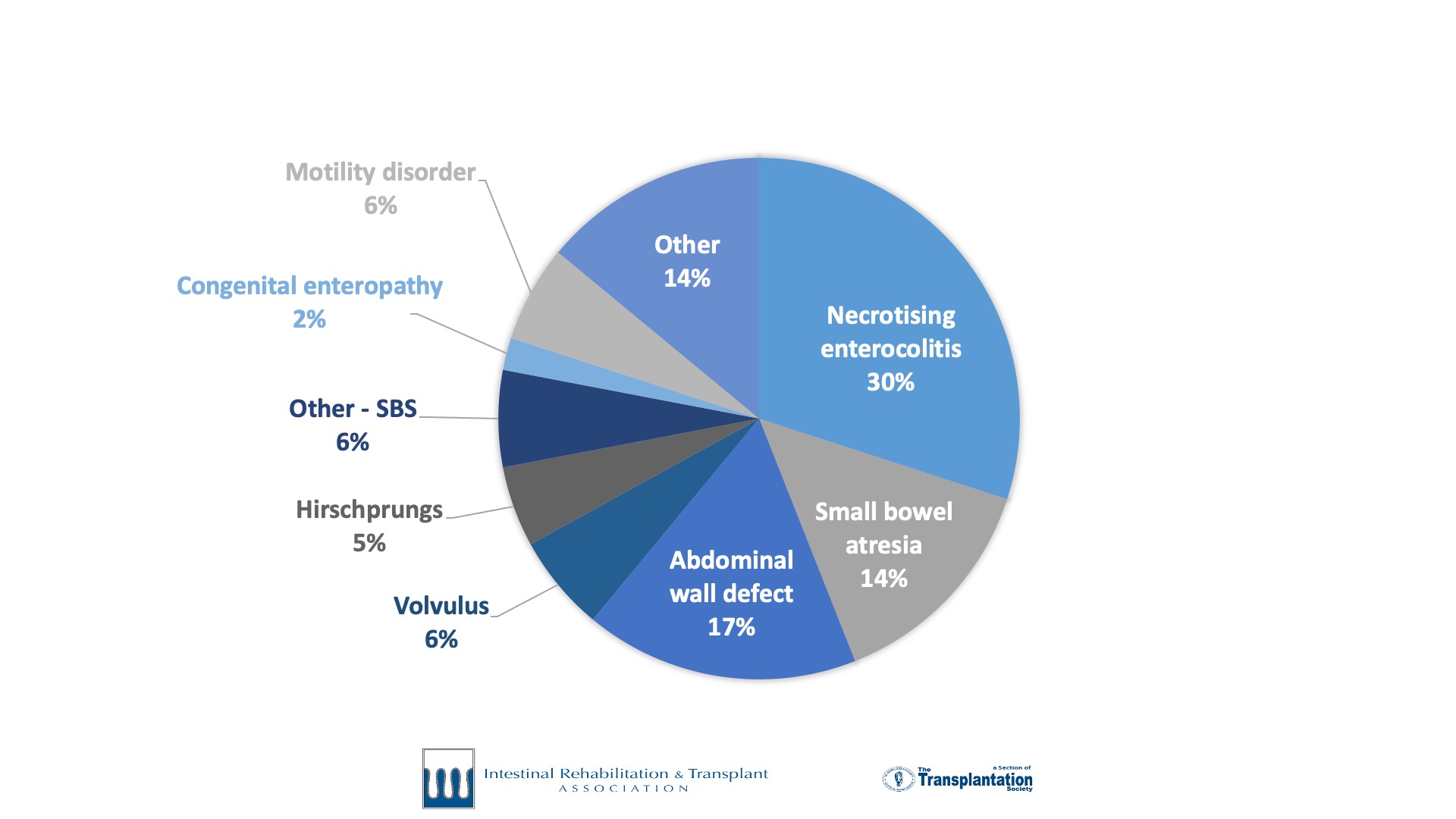
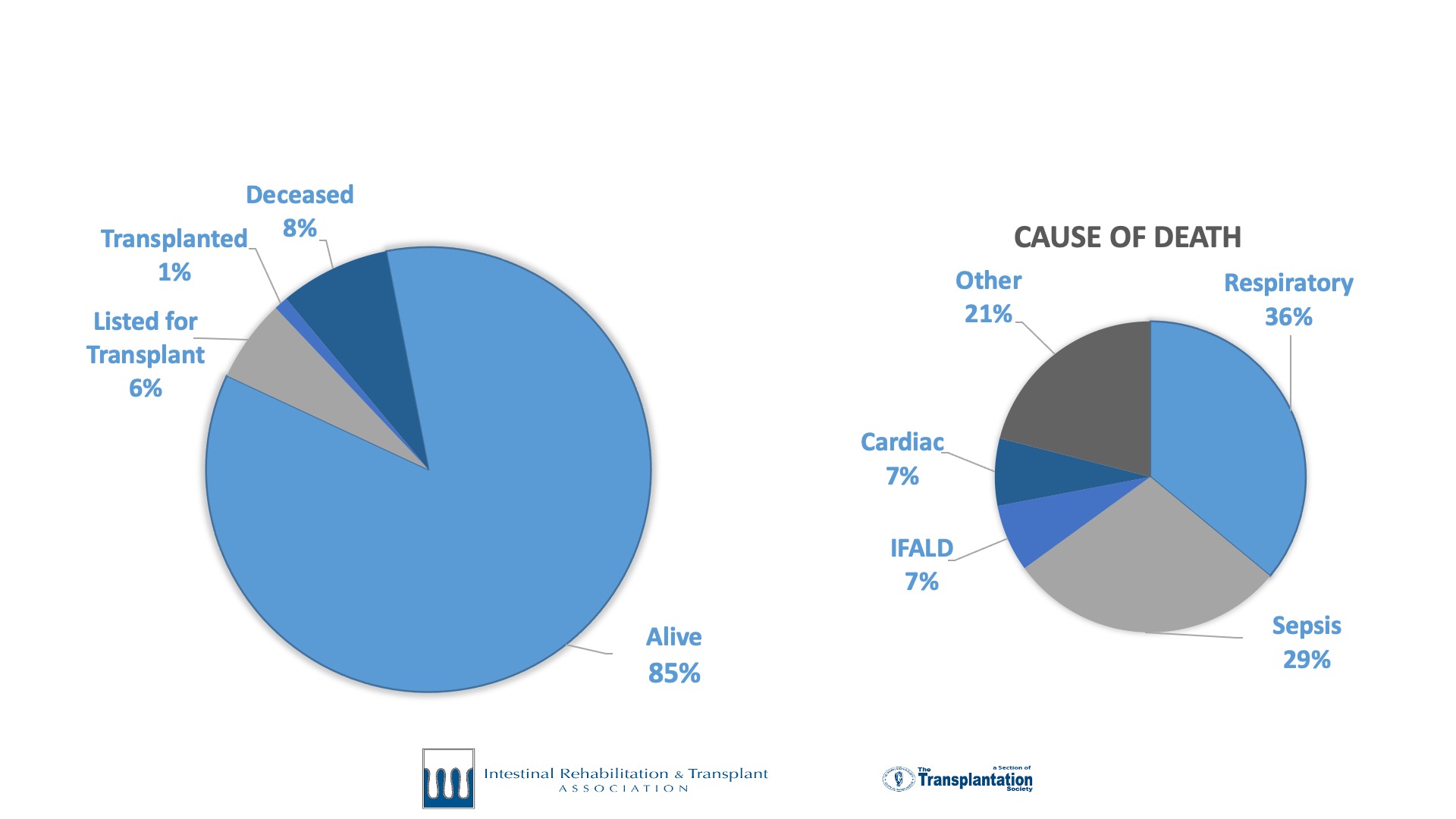
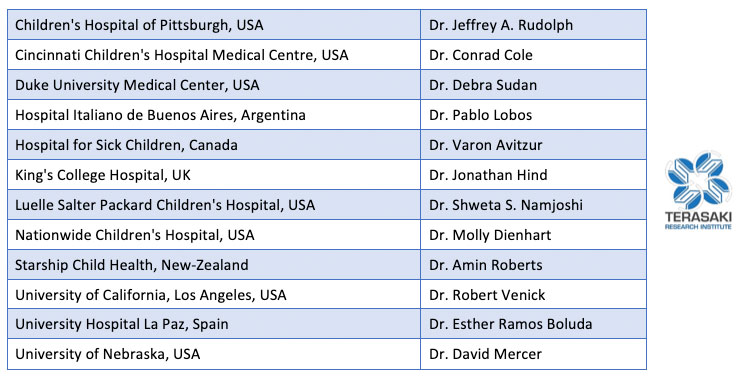
International Intestinal Failure Registry
The IIFR is endorsed by the North American Society for Pediatric Gastroenterology and Nutrition (NASPGHAN) and the American Society for Parenteral and Enteral Nutrition (ASPEN). The IIFR is supported by non-restricted and education grants by Takeda LTD and Stanford University & the Lucile Packard Children’s Hospital.

Social
Contact
Address
International Intestinal Rehabilitation & Transplant Association
c/o The Transplantation Society
740 Notre-Dame Ouest
Suite 1245
Montréal, QC, H3C 3X6
Canada